Abstract
Background:
High dose melphalan followed by autologous stem cell transplant (ASCT) remains the standard of care for transplant-eligible patients with newly diagnosed multiple myeloma (NDMM). Achievement of complete response (CR) and Minimal Residual Disease (MRD) negativity are associated with improved progression-free survival (PFS) and overall survival (OS). With superior triplet and quadruplet-based induction regimens, a higher proportion of patients are achieving deep responses of at least a VGPR or better. The probability of achieving different levels of deeper hematological responses post-ASCT based on the pre-ASCT depth of response is less clear in the existing literature but would be of value to patients and providers in discussing the added benefit of ASCT.
Objective:
We assessed the rate of deepening the hematological response with upfront ASCT in patients with NDMM, mainly to MRD negative CR, based on the response achieved after induction therapy.
Study Design:
We retrospectively reviewed 210 patients with NDMM who underwent upfront ASCT at Mayo Clinic Rochester from May 1st, 2018 to July 31st, 2019. In addition to the availability of next generation flow cytometry testing for MRD status, that yielded a sensitivity of 10-5, the more sensitive mass spectrometry-based assessment of peripheral blood (i.e., Mass-fixation) for monoclonal proteins was utilized rather than conventional immunofixation.
Results:
Pre-ASCT, 23 patients (11%) achieved MRD negative CR which increased to 66 (31%) patients post ASCT. Of 187 patients not in MRD negative CR pre-ASCT, 45 (24%) converted to MRD negative CR. Patients with MRD positive CR before ASCT had the highest rates of conversion to MRD negative CR. HR cytogenetics did not impact rates of MRD negative CR achievement post ASCT irrespective of pre-ASCT IMWG response (p = 1.0). Overall, irrespective of IMWG response, 43 (20%) patients were MRD negative pre-ASCT (19 in VGPR, 24 in CR or sCR) and 102 (49%) patients were MRD negative post-ASCT (36 in VGPR, 66 in CR or sCR). Among 85 patients with VGPR post-ASCT, 36 achieved MRD negativity of which 8 (22%) progressed, while 49 had MRD positive disease of which 24 (49%) progressed (p = 0.014).
Conclusion:
Upfront ASCT in patients with NDMM leads to deeper responses with 24% converting to MRD negative CR and more than doubling of the total rate of MRD negativity irrespective of IMWG response depth.
Keywords: Minimal residual disease, autologous stem cell transplant, Mass-Fix, multiple myeloma
INTRODUCTION
High dose melphalan followed by autologous stem cell transplant (ASCT) remains the standard of care for eligible patients with newly diagnosed multiple myeloma (NDMM) despite multiple advancements in therapy to include the incorporation of proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs) in the induction regimens 1–3. Multiple clinical trials have demonstrated the benefit of ASCT in improving the depth of hematologic responses after induction therapy, including anti-CD38 monoclonal antibodies, for patients with NDMM 4–8. ASCT is consistently associated with improved progression-free survival (PFS), but the benefit in overall survival (OS) has been less consistent or not as obvious 4,5.
Recently, minimal residual disease (MRD) negativity, with sensitivity of at least 1 in 10−5, by next generation flow (NGF) cytometry has been incorporated into the International Myeloma Working Group (IMWG) consensus criteria for response 9. MRD negativity has been shown to be associated with improved time to progression (TTP), PFS and OS in multiple clinical trials and real-world evidence 10–13. Mass spectrometry-based serum monoclonal protein detection (i.e. Mass-Fixation) has emerged as a more sensitive replacement for conventional immunofixation (IFE) 14. Thus, the achievement of MRD negative CR by utilizing both NGF and Mass-Fixation is the deepest hematological response achievable based on the availability of these next-generation technologies in clinical practice 9,14. However, a gap in the literature exists in what proportion of patients undergoing an early ASCT achieve a MRD negative CR. Thus, this study conducted a retrospective case series of all NDMM patients to assess their pre-ASCT and post ASCT-depth of hematological response.
METHODS
The electronic medical records of all consecutive patients with NDMM who underwent an upfront ASCT at Mayo Clinic Rochester from May 1st, 2018 to July 31st, 2019 were retrospectively reviewed. The study was conducted in accordance with the institutional guidelines with institutional review board approval and in accordance with the principles of the Helsinki Declaration. Upfront ASCT was defined as ASCT within one year of diagnosis. We excluded patients that did not have a hematological response assessment performed either prior to ASCT or within 100 days post-ASCT. Fluorescent in situ hybridization (FISH) assay was used to risk-stratify patients as either high risk (HR) or standard risk (SR) cytogenetics as per the Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) guidelines (https://www.msmart.org). HR patients had any of the following genetic abnormalities: t(4;14), t(14;16), t(14;20), deletion 17p or p53 mutation, and/or gain 1q.
Response assessment was based on the International Myeloma Working Group’s (IMWG) 2016 consensus criteria 9. However, instead of using IFE, a more sensitive immunoglobulin enrichment-coupled with matrix-assisted laser desorption ionization time-of-flight mass-spectrometry (MALDI-TOF), i.e., Mass-Fix, was used; Patients with Mass-Fix negative results were classified as having a complete response (MF-CR) and patients with a 90% reduction or greater but detectable monoclonal protein on Mass-Fix were classified as having a very good partial response (MF-VGPR). Additionally, to isolate the effect of ASCT on response, only the response within 100 days of ASCT were considered rather than relying on two consecutive results. Imaging as well as bone marrow aspiration and biopsy were done at the time of stem cell harvest and within 100 days post-ASCT. MRD was assessed in the bone marrow aspirate using the established Euro Flow protocol with sensitivity of 10−5. Per institutional practice, NGF-based MRD testing was reflexively performed only if screening flow cytometry, with a sensitivity of 1 in 10−4, was negative for clonal plasma cells.
The primary outcome of the study was the rate of post-ASCT conversion to MRD negative MF-CR based on the pre-ASCT depth of hematological response. Secondary outcomes include the overall percentage of post-ASCT conversion from MRD positive to MRD negative status regardless of IMWG response and the impact of HR cytogenetics on probability of conversion to MRD negativity, and TTP. In our institution all patients with NDMM receive maintenance therapy post-ASCT. Patients with SR cytogenetics receive IMiD-based maintenance therapy starting at day 100 and patients with HR cytogenetics receive combination IMiD-PI maintenance at day 60. Patient demographics are presented as median with range for continuous variables and frequency with percentages for categorical variables. Statistical analysis was performed using the SAS biostatistical software JMP 16.0.1 (SAS Institute Inc., Cary, NC). Differences between sub-groups were compared by using either the Chi-square test or Fisher exact test. Kaplan-Meier analysis was used to analyze and create TTP curves, and log rank test was used to compare subgroups.
RESULTS
A total of 210 patients were included in this study cohort of which 126 (60%) were male with a median age of 62 years (range 33–77 years). Four (3%) patients were older than 75 years. Of the 178 patients with FISH cytogenetics available at baseline, there were 78 (44%) with HR cytogenetics. The median follow-up post-ASCT was 33 months (range 1.6-45 months). All patients received induction therapy containing at least a PI, 171 (81%) patients received a combination of a PI and immunomodulator together and 13 (6%) received a quadruplet regimen containing a PI, immunomodulator and anti-CD38 monoclonal antibody.
The median time from diagnosis to ASCT was 6 months (range 3-12 months). Based on standard IMWG response criteria prior to ASCT but after induction therapy, 23 (11%) had stringent MF-CR (sCR), 27 (13%) had MF-CR, 85 (40%) had MF-VGPR, 59 (28%) had PR, 12 (6%) had MR and 4 (2%) had SD. Regardless of IMWG response, 49 (23%) patients achieved MRD negative status, of which 26 (53%) were in a MF-VGPR and 23 (47%) patients were in a MF-CR or MF-sCR. In the subsequent post-ASCT assessment, response depth increased to 66 (31%) with MF-sCR, 25 (12%) MF-CR, 85 (40%) MF-VGPR, 30 (14%) PR, and 4 (2%) MR. Regardless of standard IMWG response, 102 (49%) patients achieved MRD negative status, of which 36 (35%) were in a VGPR and 66 (65%) patients were in a MF-CR or MF-sCR.
The proportion of patients in MRD negative MF-CR increased from 23 (11%) patients in the pre-ASCT setting to 66 (31%) patients in the post-ASCT setting (Figure 1). Two patients who were in a MRD negative MF-CR at the pre-ASCT time point were not MRD negative post-ASCT but were still in MF-CR. Of the 187 patients not in MRD negative MF-CR pre-ASCT, 45 (24%) converted to MRD negative MF-CR. Table 1 depicts the percent conversion to MRD negative MF-CR post-ASCT categorized by the best IMWG response achieved pre-ASCT. Patients with MRD positive MF-CR had the highest rate of conversion to MRD negative MF-CR, with 21/27 (78%) patients converting. In patients with MF-VGPR pre-ASCT, 20 (11%) were MRD negative, of which 6 (30%) converted to MRD negative MF-CR. Of the remaining 65 (35%) patients who were in a MF-VGPR and MRD positive, 13 (20%) converted to MRD negative MF-CR. Finally, patients in less than a MF-VGPR (i.e., PR, MR or SD) had the lowest likelihood of converting to MRD negative MF-CR (5/75 or 7%). There was no difference in the proportion of patients in a MRD negative MF-CR pre-ASCT based on baseline FISH cytogenetics (SR: 8 patients or 8% vs. HR: 7 patients or 9%). Similarly, when considering baseline FISH cytogenetics and its effect on the probability of converting to MRD negative MF-CR post-ASCT, there was no difference between SR and HR patients, i.e., of the 71 patients with HR cytogenetics who were not in a MRD negative MF-CR, 16 (23%) converted to MRD negative MF-CR post ASCT, and of the 92 patients with SR cytogenetics who were not in a MRD negative MF-CR, 21 (23%) converted to MRD negative MF-CR post ASCT.
Figure 1:
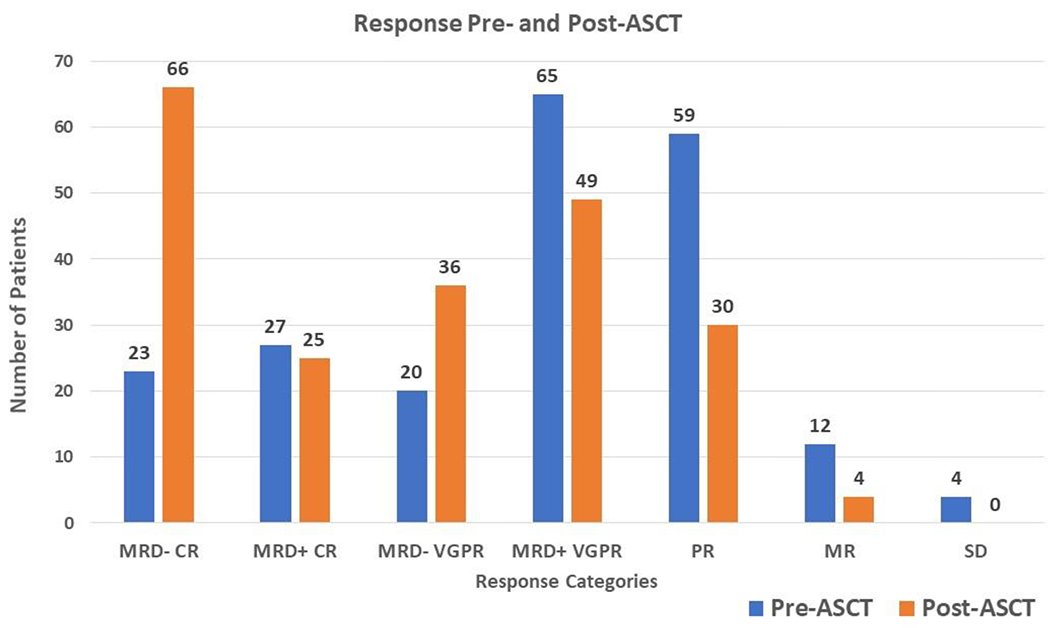
Distribution of patients among the different response categories pre and post ASCT.
Table 1.
Percent conversion to MRD− CR post-ASCT by IMWG response achieved pre-ASCT
| Pre-ASCT IMWG response | N (%) | Post-ASCT MRD(−) CR, N (%) |
|---|---|---|
| MF-CR MRD(−) | 23 (11) | 21 (91)** |
| MF-CR MRD(+) | 27 (14) | 21 (78) |
| MF-VGPR MRD(−) | 20 (11) | 6 (30) |
| MF-VGPR MRD(+) | 65 (35) | 13 (20) |
| PR | 59 (32) | 4 (7) |
| MR | 12 (6) | 1 (8) |
| SD | 4 (1) | 0 (0) |
Percent(%) in column 3 represents proportion of patients converting in each category (row)
Two patients lost MRD negative status after ASCT but remained in CR
At the time of analysis, 70 (33%) patients had evidence of disease progression and 25 (12%) patients died. In the post-ASCT setting, the median TTP was not reached for those patients who achieved MRD negative status in the bone marrow independent of IMWG response compared to a median 34 months for those who were MRD positive (p = 0.027) (Figure 2). The median OS was not reached for either group. There was no difference in TTP between those patients who were in a MRD negative MF-CR compared to those who were in a MRD positive CR (40 months vs. not reached, p = 0.43). Among 85 patients who were in a MF-VGPR post ASCT, of 36 who achieved MRD negativity, 8 (22%) progressed, while out of the 49 MF-VGPR who had MRD positivity, 24 (49%) progressed (p = 0.014). The median TTP for the patients with MF-VGPR and MRD negative status was not reached compared to 30 months for those who were MF-VGPR and MRD positive (p = 0.042) (Figure 3). Median OS was not reached for either group. Figure 4 compares the TTP outcomes post-ASCT by combining both MRD status in the bone marrow (positive, negative) and IMWG response (VGPR, ≥CR) and as patients with a MRD negative MF-VGPR have similar outcomes to those who are in a MF-CR, without regard to MRD status, but superior to those patients with a MRD positive MF-VGPR or less.
Figure 2:
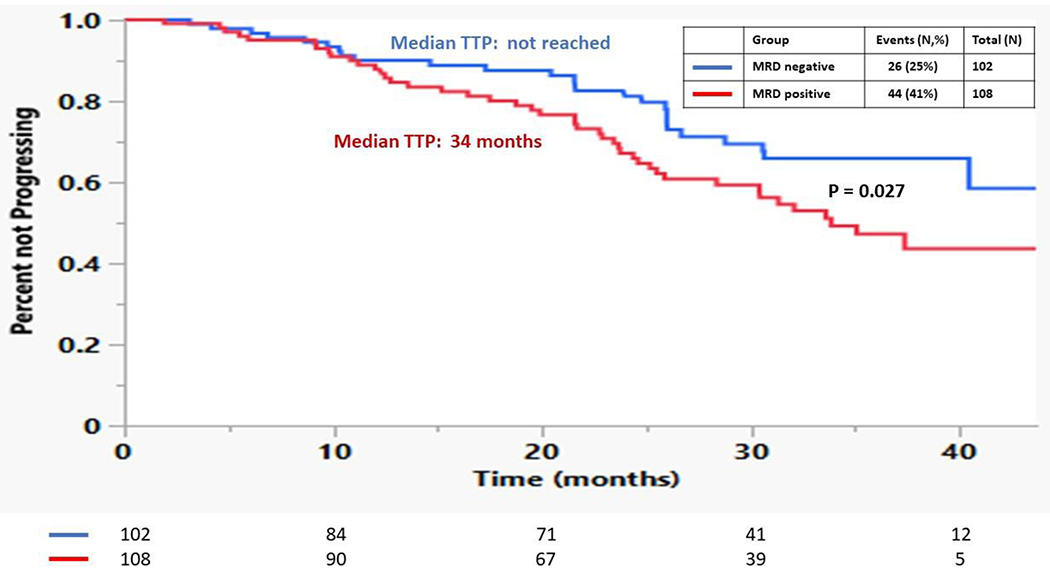
Kaplan Meier curves comparing the TTP for patients based on achieving MRD positive vs. MRD negative disease post-ASCT irrespective of IMWG response.
Figure 3:
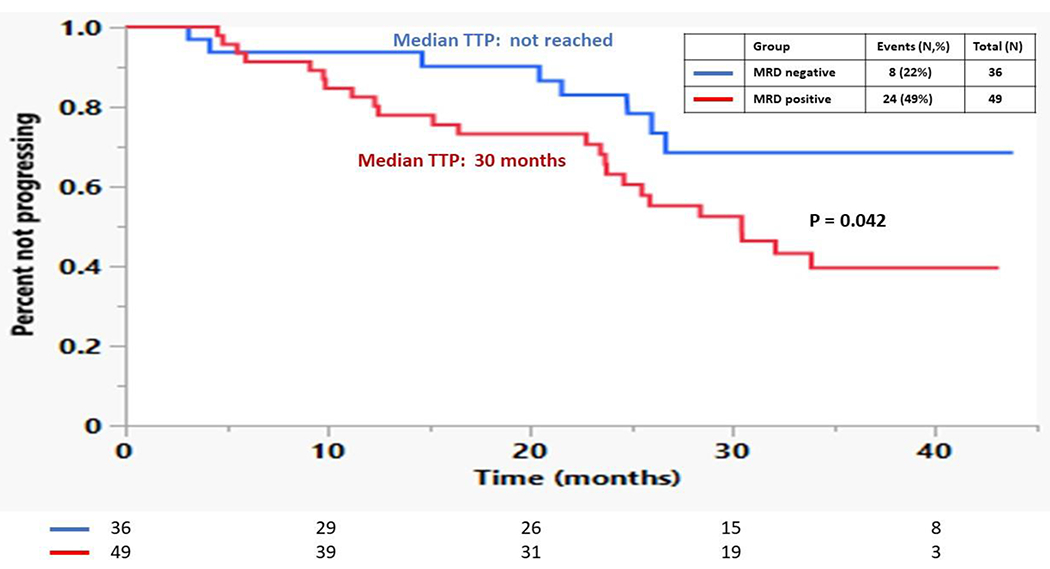
Kaplan Meier curves comparing the TTP for patients based on achieving a MF-VGPR with either MRD positive vs. MRD negative disease post-ASCT.
Figure 4:
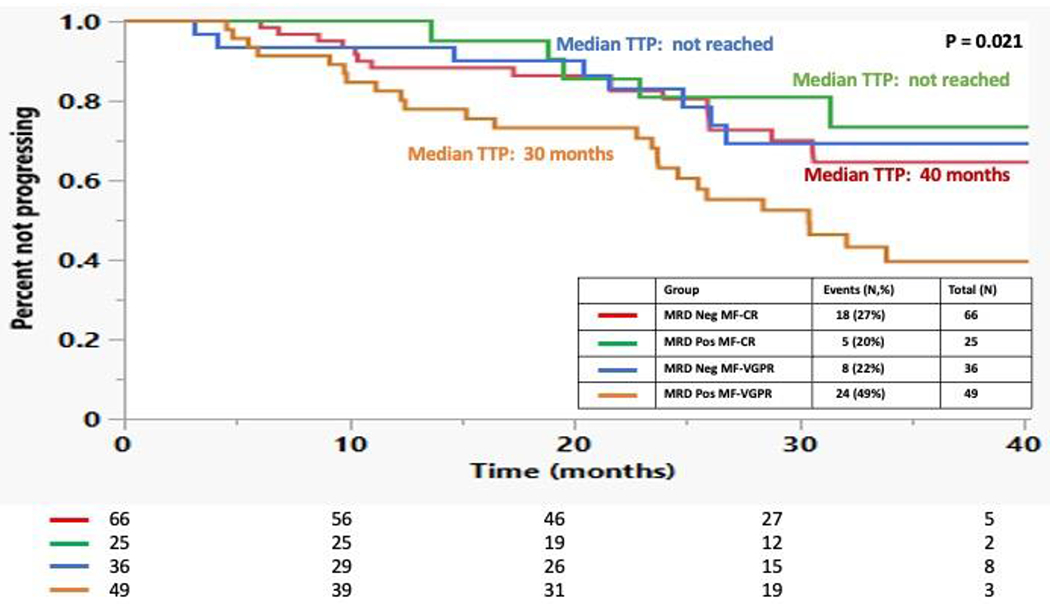
Kaplan Meier curves comparing the TTP for patients based on achieving a MRD negative MF-CR vs. MRD positive MF-CR vs. MRD negative MF-VGPR vs. MRD positive MF-VGPR or worse post-ASCT.
DISCUSSION
Our study reaffirms the benefit of an upfront ASCT by demonstrating an increased ORR and depth of response in real world patients who received induction with novel agents used in mostly triplet combinations. All the patients had at least a MR after ASCT, with the proportion of patients in MF-VGPR or better increasing from 64% to 84% and MRD negativity increasing from 23% to 49%.
Negative IFE is one of the distinguishing criteria between CR and VGPR 9; At Mayo Clinic, the more sensitive Mass-Fix is used instead of 1970’sIFE leading to a higher proportion of hematologic responses being classified as a VGPR rather than CR due to its higher sensitivity 14. Recently, MRD assessment has been incorporated into the IMWG response assessment criteria 9 and MRD negativity correlates with longer PFS and OS 9. Currently, MRD negative MF-CR is the deepest response attainable. In our patients with NDMM undergoing an upfront ASCT, the proportion of patients in MRD negative MF-CR rose from 11% to 31% post-ASCT. Our paper outlines the rates of conversion to MRD negative MF-CR based on the pre-ASCT response, with 78% converting from MRD positive MF-CR, 30% from MRD negative MF-VGPR, 20% from MRD positive MF-VGPR, and less than 10% in patients with PR or less. These results need to be validated by a larger sample and the use of Mass-Fix lowers the rate of conversion compared to standard IFE. Nonetheless, the proportion of patients converting from each response category may be useful for patient-informed decision making and expectation management, especially as discrepancies between patient and physician expectations of transplant outcomes have been demonstrated 15.
Interestingly, patients with HR cytogenetics had similar rates of MRD negative MF-CR achievement after induction as well as post-ASCT conversion in our cohort, highlighting the benefit of ASCT in patients with NDMM regardless of baseline cytogenetic risk. Two patients in MRD negative MF-CR pre-ASCT remained in MF-CR but were MRD positive post ASCT, emphasizing the limitations of MRD assessment including the possibility of sampling errors or dilute samples obtained pre transplant. 16. This highlights the added prognostic value of sustained MRD negativity, which has been previously documented 17,18.
Although MRD negative MF-CR is the deepest response possible, there was no difference in the TTP between MRD positive and MRD negative patients in MF-CR in contrast to previous data by the PETHEMA group 19. However, similar to the data by the PETHEMA group, TTP in patients with MRD negative MF-VGPR was like the TTP in patients with a MRD negative MF-CR, whereas patients with MRD positive MF-VGPR had significantly shorter TTP. The discrepancies between studies could be due to the relatively short median follow up in our study. TTP was longer in patients who achieved MRD negativity regardless of standard IMWG response criteria (NR vs. 34 months, p=0.027) and this difference was also observed in patients with MRD negative MF-VGPR (NR vs. 30 months, p=0.042) where the rate of disease progression was higher in the MRD positive group compared to the negative group (22% vs. 49%. P=0.014). This difference was not observed in patients in MF-CR, with TTP being similar among patients with MRD positivity and negativity. However, this may be related to the increased sensitivity of Mass-Fix, with patients achieving a MF-VGPR who might have otherwise been labelled as a CR if traditional IFE was done 14,20. With increased sensitivity of Mass-Fix, up to 10 times greater than IFE 21, achievement of a MF-CR indicates a very deep response and the added prognostic value of MRD negativity was lost, albeit with a short follow up. The enhanced prognostic utility of serum Mass-Fix may supplant MRD negativity in the future, especially considering the ease of collecting serial serum samples compared to bone marrow samples.
Limitations of this study include short follow up and small sample size. Incorporation of MRD as part of the routine response assessment in MM was recent and data was available for only a small sample of patients with a short median duration of follow up. The heterogeneity in maintenance therapy post-ASCT could also affect the TTP outcomes observed in our cohort. Despite these limitations, this is the first study to provide the rates of conversion with upfront ASCT to the deepest responses in patients with NDMM based on pre-ASCT response. This carries important implications from both patient and clinician perspectives, for informed decision making. Longer follow up to assess PFS and OS is still needed, especially in the evolving landscape of multiple myeloma treatment, with some studies assessing CAR-T rather than ASCT consolidation and other studies investigating the role of therapy intensification or de-escalation based on MRD status.
Supplementary Material
Highlights:
Upfront ASCT more than doubled the rate of MRD negativity in patients with NDMM
Patients with MRD+ CR were the most likely to achieve MRD− CR
MRD negativity predicted improved OS and TTP, especially when Mass-Fix was positive
MRD negativity didn’t affect TTP when Mass-Fix was negative (i.e. MF-CR)
ACKNOWLEDGEMENTS
Research reported in this publication was supported by Mayo Clinic Hematological Malignancies Program and in part by grants from the National Cancer Institute of the National Institutes of Health under Award Number R01 CA254961 (W.I.G). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Finally, this research is also supported in part by generous funding from philanthropic donations to the Mayo Clinic.
Footnotes
Conflict-of-interest disclosure:
These authors declare no competing financial interests.
REFERENCES
- 1.Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer Journal. 2020;10(9):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikhael J, Ismaila N, Cheung MC, et al. Treatment of multiple myeloma: ASCO and CCO joint clinical practice guideline. Journal of Clinical Oncology. 2019;37(14):1228–1263. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2021;32(3):309–322. [DOI] [PubMed] [Google Scholar]
- 4.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. New England Journal of Medicine. 2017;376(14):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gay F, Musto P, Rota-Scalabrini D, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. The Lancet Oncology. 2021;22(12):1705–1720. [DOI] [PubMed] [Google Scholar]
- 6.Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. The Lancet. 2019;394(10192):29–38. [DOI] [PubMed] [Google Scholar]
- 8.Costa LJ, Chhabra S, Callander NS, et al. Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone (Dara-KRd), Autologous Transplantation and MRD Response-Adapted Consolidation and Treatment Cessation. Final Primary Endpoint Analysis of the Master Trial. Blood. 2021;138:481. [Google Scholar]
- 9.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The lancet oncology. 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 10.Paiva B, Gutiérrez NC, Rosiñol L, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood, The Journal of the American Society of Hematology. 2012;119(3):687–691. [DOI] [PubMed] [Google Scholar]
- 11.Paiva B, Vidriales M-B, Cerveró J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood, The Journal of the American Society of Hematology. 2008;112(10):4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540–2547. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood, The Journal of the American Society of Hematology. 2014;123(20):3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray DL, Puig N, Kristinsson S, et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer Journal. 2021;11(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Fairclough D, Antin JH, Weeks JC. Discrepancies Between Patient and Physician Estimates for the Success of Stem Cell Transplantation. JAMA. 2001;285(8):1034–1038. [DOI] [PubMed] [Google Scholar]
- 16.Landgren O MRD testing in multiple myeloma: from a surrogate marker of clinical outcomes to an every-day clinical tool. Seminars in Hematology: Elsevier; 2018:1–3. [DOI] [PubMed] [Google Scholar]
- 17.Avet-Loiseau H, San-Miguel J, Casneuf T, et al. Evaluation of sustained minimal residual disease negativity with daratumumab-combination regimens in relapsed and/or refractory multiple myeloma: analysis of POLLUX and CASTOR. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2021;39(10):1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman JL, Laubach JP, Sborov D, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated analysis of griffin after 12 months of maintenance therapy. Blood. 2020;136:45–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiménez-Ubieto A, Paiva B, Puig N, et al. Validation of the International Myeloma Working Group standard response criteria in the PETHEMA/GEM2012MENOS65 study: are these times of change? Blood. 2021;138(19):1901–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandakumar B, Murray DL, Dispenzieri A, et al. Sequential Comparison of Conventional Serum Immunofixation (IFE) to Mass Spectrometry-Based Assessment (MASS FIX) in Patients with Multiple Myeloma (MM). Blood. 2020;136:12–13. [Google Scholar]
- 21.Eveillard M, Rustad E, Roshal M, et al. Comparison of MALDI-TOF mass spectrometry analysis of peripheral blood and bone marrow-based flow cytometry for tracking measurable residual disease in patients with multiple myeloma. British journal of haematology. 2020;189(5):904–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


