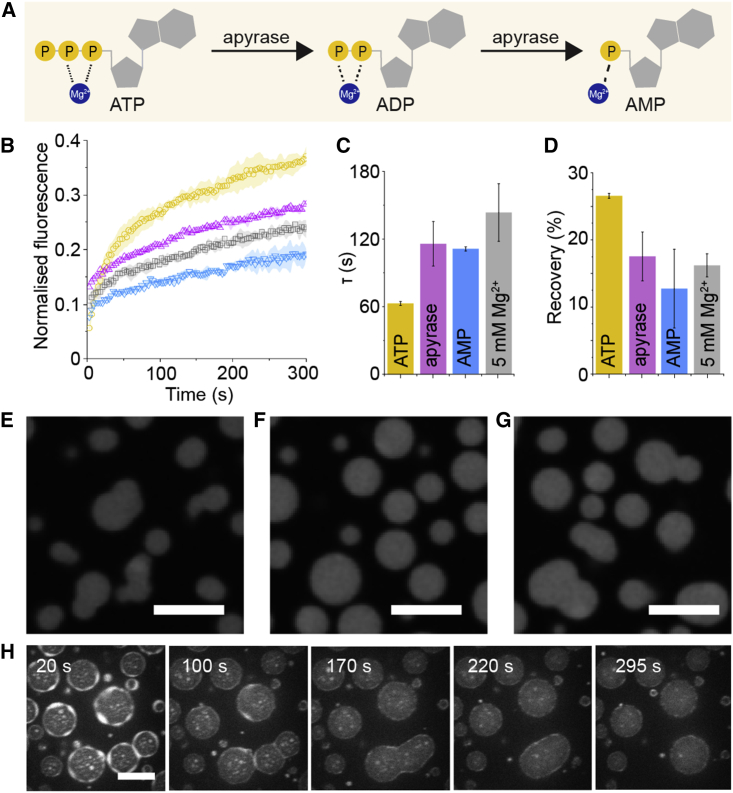
Figure 4.
Enzymatic control of ATP concentrations influences condensate morphology and dynamics. (A) Apyrase enzymes catalyze the removal of phosphate groups from ATP to form AMP, a nucleotide that poorly chelates Mg2+. (B–D) The effect of apyrase on the dynamics of rRNA after FRAP is clearly shown, where rRNA recovery in the presence of ATP (yellow) drops when apyrase (purple) is added. (C and D) In fact, the resulting FRAP parameters show that apyrase converts ATP to AMP, which imbues the rRNA with gel-like dynamics, similar to condensates made in 5 mM Mg2+ buffer (grey). (E–G) The morphology of NPM1-rRNA condensates at 18°C in 5 mM Mg2+ buffer (E) with 5 mM ATP added (F) and when apyrase is also added (G) shows the changes in morphology that is expected from gel-like condensates in buffer containing Mg2+ that stabilizes RNA-RNA interactions and spherical morphology in conditions where ATP chelates the Mg2+ and liquefies these interactions. (H) This series of confocal images over time of labeled 70S ribosomes shows the disappearance of the ribosome halo and puncta as apyrase was added. ATP depletion results in higher Mg2+, which stabilizes the ribosomes and causes them to dissipate back into the dilute phase. The errors in this figure are standard deviations from triplicate measurements. Scale bars are all 10 μm. To see this figure in color, go online.