Abstract
Insulin is a mainstay of therapy for diabetes mellitus, yet its thermal stability complicates global transportation and storage. Cold-chain transport, coupled with optimized formulation and materials, prevents to some degree nucleation of amyloid and hence inactivation of hormonal activity. These issues hence motivate the design of analogs with increased stability, with a promising approach being single-chain insulins (SCIs), whose C domains (foreshortened relative to proinsulin) resemble those of the single-chain growth factors (IGFs). We have previously demonstrated that optimized SCIs can exhibit native-like hormonal activity with enhanced thermal stability and marked resistance to fibrillation. Here, we describe the crystal structure of an ultrastable SCI (C-domain length 6; sequence EEGPRR) bound to modules of the insulin receptor (IR) ectodomain (N-terminal α-subunit domains L1-CR and C-terminal αCT peptide; “microreceptor” [μIR]). The structure of the SCI-μIR complex, stabilized by an Fv module, was determined using diffraction data to a resolution of 2.6 Å. Remarkably, the αCT peptide (IR-A isoform) “threads” through a gap between the flexible C domain and the insulin core. To explore such threading, we undertook molecular dynamics simulations to 1) compare threaded with unthreaded binding modes and 2) evaluate effects of C-domain length on these alternate modes. The simulations (employing both conventional and enhanced sampling simulations) provide evidence that very short linkers (C-domain length of −1) would limit gap opening in the SCI and so impair threading. We envisage that analogous threading occurs in the intact SCI-IR complex—rationalizing why minimal C-domain lengths block complete activity—and might be exploited to design novel receptor-isoform-specific analogs.
Significance
Homologous to single-chain insulin-like growth factors, single-chain insulins (SCIs) represent a platform for design of ultrastable therapeutic analogs. Promising to circumvent the complex and costly cold chain of insulin distribution (a key challenge in global health), SCIs as a class exhibit marked resistance to fibrillation at or above room temperature. How SCIs bind to the insulin receptor is, however, not well understood. We present an x-ray crystal structure of an SCI bound to a domain-minimized fragment of the receptor. The structure is remarkable for penetration of a receptor-derived peptide through the SCI, like a thread through a needle’s eye. This novel feature, further probed through biochemical studies and molecular dynamics simulations, rationalizes why highly constrained SCIs are without biological activity.
Introduction
Single-chain insulin (SCI) analogs represent a promising therapeutic platform for the treatment of diabetes mellitus, owing to their low mitogenicity and marked resistance to chemical and physical degradation at or above room temperature (1, 2, 3). The latter biophysical properties might facilitate global distribution by circumventing costly and complex cold-chain logistics (4,5). Similar in core structure to native insulin, SCIs mimic the conventional domain organization of insulin-like growth factors (IGFs) and insulin’s biosynthetic precursor proinsulin (Fig. 1). The latter consists of a B domain (30 residues) and A domain (21 residues) connected by a C domain (35 residues in human proinsulin) (6,7) that acts as a flexible tether (8) between the helical elements of the B and A domains of the hormone (9). Vertebrate insulins and IGFs share a common evolutionary protochordate ancestor (10). Early SCI-related analogs were prepared using short bifunctional chemical cross-linking reagents, typically connecting the ε-amino group of LysB29 to the α-amino group of GlyA1 (11,12). Such analogs demonstrated a negative correlation between linker length and receptor affinity (11,13). Similarly, connecting the C-terminus of the B chain to the N-terminus of the A chain, either via a direct B30-A1 peptide bond or via an intervening peptide (foreshortened C domain), demonstrated the functional importance of intramolecular flexibility (3).
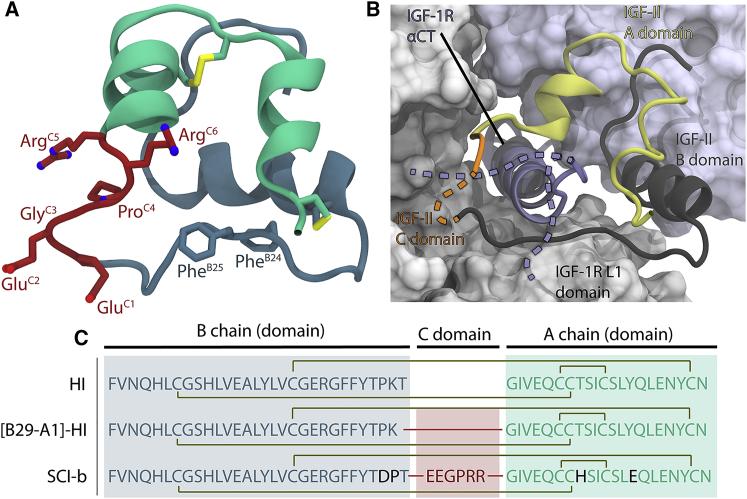
Figure 1.
Structural overview of insulin analogs and sequences. (A) Solution structure of SCI-b (PDB: 5WBT) with A, B, and C domains shown in green, blue, and red, respectively. Side chains of essential receptor-engaging residues PheB24 and PheB25 along with all C-domain residues and disulfide bonds (in yellow) are shown in stick representation. (B) Cryo-EM structure of IGF-II bound to the IGF-1R ectodomain (PDB: 6VWI). The IGF-II A domain is shown in yellow, B domain in black, and C domain in orange. The entire C domain is not visible within the structure; the presumed location of its missing residues is indicated (dashed orange). Although the complex is indicative of a threaded IGF-1R αCT domain (purple), the two alternate “threaded” and “unthreaded” hypothetical versions of the IR-B isoform αCT domain as it would relate to an SCI on the IR are depicted (dashed purple). (C) Protein sequences of native human insulin (HI), an SCI with a 1-residue C domain ([B29-A1]-HI), and the previously presented SCI-b (1,2). In all sequences, the A domain (or A chain) is shown in green, the B domain (or B chain) in blue, and the connecting C domain in red. Substitutions relative to native HI are shown in black. The red lines connecting the A and B domains to the C domain indicate peptide bonds, with dark-yellow lines indicating disulfide bonds. To see this figure in color, go online.
This foundational work foreshadowed the present understanding of how insulin alters its conformation to bind and activate the insulin receptor (IR) (for reviews, see (14) and (15)). The human IR (hIR) is a membrane-bound disulfide-linked homodimer; each protomer contains an α- and β-subunit (16). Human insulin (HI) engages several receptor domains across two distinct sites, designated “site 1” and “site 2” (and dimer-related sites 1ʹ and 2ʹ). On binding of the hormone to high-affinity site 1 (≈6.4 nM), the insulin molecule undergoes significant B-chain reorganization. The hormone’s C-terminal β-strand detaches from the α-helical core and pivots away from the B-chain central α-helix, exposing conserved nonpolar side chains otherwise inaccessible within the core (17,18). Such “opening” of the hormone enables, in particular, engagement of conserved aromatic residues in insulin with both the hIR first leucine-rich repeat domain (L1) and the C-terminal region of an α-helical domain formed by the alternate receptor α-chain (αCTʹ), as well as engagement of the hormone with the membrane-distal loops of domain FnIII-1ʹ (19). Recent cryo-electron microscopy (cryo-EM) studies of HI bound to the intact IR ectodomain and holoreceptor under ligand-saturated conditions (20, 21, 22, 23, 24) describe the mechanism by which the hormone also binds to the canonical ABE β-sheet of domain FnIII-1, that of HI binding site 2. The relationship of this site to early kinetic and complex negative cooperativity studies (25,26) remains, however, unclear.
Ascribed to the lower-affinity site 2 (≈400 nM) (17,19), the hormone engages the FnIII-1 domain via residues of the hormone spatially opposite to those which participate in the binding to site 1. Notably, binding of HI to site 2 does not necessitate the internal C-terminal B-chain reorganization essential in binding site 1 (20, 21, 22, 23, 24). The recent cryo-EM studies provide hypotheses describing the sequential binding of HI from site 2′ to site 1; however, information describing the molecular transition states underlying the large interprotomer motion with either saturated or unsaturated insulin binding is not yet fully resolved.
The present study exploits a domain-minimized model of the hormone-receptor complex to focus on site 1, envisioned as the trigger for allosteric receptor reorganization (17,27). This model, designated the microreceptor (μIR), contains two fragments of the hIR α-subunit: 1) domain L1 and adjoining cysteine-rich (CR) region (termed IR310.T (17)) and 2) separately, an α-chain C-terminal peptide (17). How an SCI might engage site 1 is not well understood owing to uncertainty regarding the orientation and location of αCT relative to the SCI C domain. Should an SCI bind analogously to two-chain insulin, the C-terminal region of the C domain would likely occupy space ordinarily occluded by αCT (28,29). To avoid such a clash, two descriptive models have been proposed, each reconciling the reorganization of these domains by analogy to how IGF binds to its cognate receptor (IGF-1R) (28,30). The first model, in which disordered αCT C-terminal residues penetrate a loop opened by displacement of the B-chain C-terminal segment, is described as “threaded” (Fig. 1 B). The second model posits that αCT is instead directed away from the C-domain loop and is described as “unthreaded” (Fig. 1 B). Although recent cryo-EM structures of IGFs bound to IR, IGF-1R, or hybrid ectodomains (Table 1) would, by analogy, favor the threaded model, this binding mode seems inconsistent with IR splicing isoform B (IR-B) (29,30), whose αCT domain is longer than that of the A isoform (IR-A) by 12 residues, inserted between IR-A residues 717 and 718 (31). Threading of this longer IR-B-specific αCT domain would likely differ in structure and kinetics, with potential relevance to IR isoform selectivity (29,30).
Table 1.
Structures of IR and IGF-1R ectodomain fragments and their single-chain ligand complexes
| Receptor | Construct | Fv | Ligand | Resolution (Å) | Technique | PDB ID | Remark |
|---|---|---|---|---|---|---|---|
| IR | IR L1-CR + IR αCT704–719 | 83-7 | SCI-b | 2.60 | crystal | 7KD6 | this work |
| IR | IR L1-CR + IR αCT704–719 | 83-7 | HI | 2.90 | crystal | 6VEP | Xiong et al., 2020 (33) |
| IGF-1R | leucine-zippered IGF-1R ectodomain | none | IGF-II | 3.21, 4.26, 3.70, 4.21 | cryo-EM | 6VWG, 6VWH, 6VWI, 6VWJ | Xu et al., 2020 (64) |
| IGF-1R | mouse IGF-1R holoreceptor | none | IGF-I | 4.30 | cryo-EM | 6PYH | Li et al., 2019 (67) |
| IGF-1R | IGF-1R ectodomain (Δβ) | 24-60 | IGF-I | 3.27 | crystal | 5U8Q | Xu et al., 2018 (63) |
| Hybrid | IR L1-CR + IGF-1R αCT691–706 | none | IGF-I | 3.00 | crystal | 4XSS | Menting et al., 2015 (28) |
| Hybrid | leucine-zippered IGF-1R ectodomain + leucine-zippered IR-B ectodomain | none | IGF-I | 3.70, 3.73 | cryo-EM | 7S0Q, 7S8V | Xu et al., 2022 (30) |
We have previously described an ultrastable and fibrillation-resistant SCI, designated SCI-b (2). This analog incorporated numerous features intended to co-optimize its biophysical, biochemical, and biological properties. The C domain of SCI-b contains six residues (sequence EEGPRR) (1,2) (Fig. 1 C); GluC1 and GluC2 are included to enhance solubility by altering the peptide isoelectric point, GlyC3 and ProC4 allows for flexibility in the backbone chain, and ArgC5 and ArgC6 mimic the charge pattern of the dibasic cleavage site at the C-to-A-domain junction in proinsulin. Preliminary modeling of SCI-b engaging the IR in a native-like and threaded manner suggested that such a mechanism is allowed in a site-1 complex and indeed may be required. The crystallographic analysis of SCI-b in a μIR complex presented here demonstrates a threaded mode of binding. The complex was further probed by molecular dynamics (MD) simulations in both threaded and unthreaded binding modes, with energetics of internal insulin motions evaluated by both conventional and enhanced sampling MD simulations. Experiments were also undertaken to compare binding of SCI-b and HI to the hIR.
Materials and methods
Preparation of insulin analogs
HI (Sigma-Aldrich, St. Louis, MO) and SCI-b were expressed in Pichia pastoris and purified as described previously (1,2).
IR310.T:Fv 83-7: Protein production and SCI-b complex formation
The endoglycosidase-H-treated μIR stabilized by an Fv module (32) (the IR310.T:Fv 83-7 complex) was obtained from the identical protein batch as that employed for crystallization of the complex with HI (33) but was prepared here at a concentration of 4.5 mg mL−1 in 10 mM HEPES at pH 7.5. The complex was then combined with 3 mole equivalents of human IR αCT704–719 peptide (GenScript, Piscataway, NJ) and 1.8 mole equivalents of synthetic SCI-b. The protein mixture (“SCI-b/μIR.Fv”) was then used to grow crystals in hanging-drop format over a reservoir holding 0.4 mL of well solution 1 (16% polyethylene glycol [PEG] 3350, 0.2 M sodium thiocyanate [NaSCN], 0.02% sodium azide [NaN3]), a condition arising from an approximately 600-condition sparse-matrix screen conducted at the CSIRO Collaborative Crystallization Center (CSIRO C3; Parkville, VIC, Australia). Drop ratios were 1 μL protein to 1 μL well solution, and the resultant crystals consisted of plates and blocks of up to 30 μm in length. A larger, single crystal was then grown by macroseeding one such block into a 0.3 μL drop of well solution 2 (15% PEG 3350, 0.2 M NaSCN, 50 mM 2-(N-morpholino)ethanesulfonic acid-NaOH [pH 7.5], 0.02% NaN3) that was placed on a fresh coverslip alongside a 1.0 μL drop of the same protein described above. These were incubated for 1 min over a well containing 0.4 mL of well solution 1 to eliminate microcrystals, and the drops were then combined. In time, the crystal grew to ≈90 μm in length.
SCI-b/μIR.Fv: X-ray diffraction data collection and processing
For cryoprotection, the above crystal was soaked in a progressive series of 0.3 μL drops containing 10%, 20%, or 30% glycerol in 18% PEG 3350 plus 0.2 M sodium thiocyanate for ≈10–20 s in each drop, followed by direct plunging in a loop into a liquid nitrogen bath. Diffraction data were collected at 100 K with an ADSC Q315R CCD detector at beamline MX2 (λ = 0.9537 Å) at the Australian Synchrotron (Melbourne, VIC, Australia) (34). Data were integrated and merged using the XDS package version 3 (November 2014) (35) to 2.6 Å resolution based on an assessment of the significance of the CC1/2 statistic (36) as reported by XDS. Data processing statistics are presented in Table 2.
Table 2.
Diffraction data collection and refinement statistics for the crystal structure of SCI-b bound to IR310.T + IR αCT704–719 co-complexed with Fv 83-7
| PDB: 7KD6 | |
| Diffraction data collection | |
| Space group | P21 |
| Cell dimensions | |
| a, b, c (Å) | 97.67, 128.43, 148.79 |
| α, β, γ (°) | 90, 90.18, 9 |
| Resolution (Å) | 45.67–2.60 (2.70–2.60)a |
| Rmerge | 0.216 (3.32)a |
| I/σ(I) | 6.1 (0.51)a |
| CC1/2 | 0.990 (0.168)a |
| Completeness (%) | 99.8 (97.6)a |
| Redundancy | 3.8 (3.8)a |
| Refinement | |
| Resolution (Å) | 44.28–2.60 |
| No. of reflections | 112,310 |
| Rwork/Rfree | 0.178/0.188 |
| No. of atoms | 19,073 |
| Protein | 18,347 |
| Carbohydrate | 561 |
| Water | 165 |
| <B> factors | |
| Protein | 87 |
| Carbohydrate | 119 |
| Water | 50 |
| RMSD from ideality | |
| Bond lengths (Å) | 0.014 |
| Bond angles (°) | 1.8 |
Values in parentheses are for highest-resolution shell. Diffraction data were collected from a single crystal.
SCI-b/μIR.Fv: Crystal structure solution and model refinement
Four copies of SCI-b/μIR.Fv packed with 222-point group symmetry were located within the crystallographic asymmetric unit employing a search model comprising a single copy of the insulin-complexed μIR in turn in complex with Fv 83-7 (derived from Protein Data Bank entry PDB: 4OGA (18)); molecular replacement was conducted with PHASER (37) within the PHENIX suite (38). The structure was then subjected to multiple rounds of model building and improvement within COOT (39) and crystallographic refinement with BUSTER v2.10.2 (40). BUSTER refinement included TLS refinement (41) and automated noncrystallographic symmetry restraints between the four assemblies (42); residues judged to deviate systematically in conformation across the four copies in the crystallographic asymmetric unit were explicitly excluded from such restraints. Local structure-similarity restraints (42) were also applied to restrain the structure to the higher-resolution structures of the IR L1-CR-L2 fragment (PDB: 2HR7, both copies (43)), the heavy-chain variable-domain component of the Fab NMC4 from PDB: 1FNS (44), the light-chain variable-domain component of Fab G3519 from PDB: 1IL1 (45), and the light-chain variable-domain component of chimeric antibody X836 from PDB: 3MBX (46). Final crystallographic refinement statistics are presented in Table 2. Ramachandran statistics are: favored region 95.4%, allowed region 4.31%, and outliers 0.27%; other statistics are: clash score 2.94 and rotamer outliers 2.9% (computed using MolProbity (47) within PHENIX v 1.16-3549-000).
Insulin receptor-binding affinity
Affinity of all analogs for the insulin minireceptor (i.e., for the hIR L1-CR-L2 module complemented with an αCT peptide) were assessed using isothermal titration calorimetry (ITC) as described previously (48). Experiments employed a MicroCal iTC200 instrument (Malvern Instruments, Malvern, UK) using the NITPIC (49) and SEDPHAT (50) software packages to fit the data to a single-site interaction (A into BC). Insulins prepared as above were buffered at 100 μM Tris-buffered saline. αCT peptides were purchased as lyophilized powders (GenScript) and prepared in 10 mM HCl prior to use. Analogs were titrated against the L1-CR-L2 module (IR485 construct (43)) buffered in Tris-NaCl buffer (25 mM Tris-HCl, 137 mM NaCl, 2.7 mM KCl, pH 8.0) produced and purified as described (48), in the presence of a 10× molar ratio of αCT (residues 704–719 of the IR-A isoform or 704–731 of the IR-B isoform). All buffers were matched within each experiment, and the concentration of HCl differed between experiments due to the different concentrations of αCT peptides and insulin analogs. In total 16 injections were made, with the initial injection of 1.00 μL and all subsequent being 2.52 μL with a time interval of 180 s. The cell was kept at 25°C and the sample contents stirred at 750 rpm. Three technical replicates were performed, with errors in KD presented as SEM.
Molecular homology modeling
Models of SCI analogs were created with the Modeller (v9) (51) utility using the ensemble structure of unbound insulin lispro (PDB: 2KJJ), and of HI in complex with the receptor L1-CR-L2 module (extracted from PDB: 6HN5) (for uncomplexed and complexed simulations, respectively). IR-complexed models included either an IR-A or IR-B isoform αCT peptide, residues Thr704–Ser719, and Thr704–Ser731, respectively, and a single N-linked N-acetyl-D-glucosamine residue at each of the hIR residues Asn16, Asn25, Asn111, Asn215, Asn255, Asn397, and Asn418 (52). Models with the five lowest Modeller objective functions were used to seed five respective replica simulations, with five models taken from an NMR ensemble (PDB: 2KJJ) used in model building to seed insulin lispro replica simulations. Modeling of SCI-b in complex with the L1-CR-L2 module with an unthreaded [IR-B]-isoform-derived αCT employed restraints to direct the C-terminal region of the peptide away from the SCI C-domain loop, facilitating models that were either partially threaded or completely unthreaded. Restraints were employed by applying a harmonic potential (single Gaussian) from the Cβ atoms of αCT peptide residues Thr704–Val715 with a mean distance of 7 Å and a standard deviation of 0.1 Å. This restraint was found to be sufficient to sample the partially threaded or completely unthreaded orientations of αCT. Of the 50 models created, the five models with the lowest Modeller objective function were subsequently used after manually removing models which were threaded.
Molecular dynamics simulations
Simulations were conducted using the Gromacs (v.2019 and v.2020) (53) suite with the Charmm36m force field (54,55). Complexed and uncomplexed simulations of insulin analogs were solvated in 60.0 Å and 95.61 Å cubic boxes, respectively, using the TIP3P single-point water model. Sodium and chloride ions were added to neutralize the systems and provide a final ionic strength of 0.1 M. Temperature coupling was achieved using velocity rescaling applying a coupling time of 0.1 ps with protein and water/ions coupled separately at 300 K, and Parrinello-Rahman (56) pressure coupling with a coupling time of 2.0 ps. Simulations were performed with a single nonbonded cutoff of 12 Å with the Verlet neighbor searching cutoff scheme updating the neighbor list every 50 fs, and with a time step of 2 fs. Periodic boundary conditions were used with the particle-mesh Ewald method to account for long-range electrostatics, a grid width of 1.0 Å, and a fourth-order spline interpolation. All bond lengths were constrained with the P-LINCS algorithm (57). Simulations were minimized initially using the steepest descent minimization protocol to a maximum of 50,000 steps, followed by simulation for 100 ps sequentially in the NVT (canonical) and NPT (isothermal-isobaric) ensembles with all protein atoms positionally restrained. All individual uncomplexed and complexed simulation replicas were allowed to continue unrestrained for 5 μs and 1 μs each, respectively.
Well-tempered and multiple-walker metadynamics
Well-tempered and multiple-walker metadynamics simulations (WTMetaD and MWMetaD, respectively) were conducted using the PLUMED (v.2.5) plugin (58) to Gromacs (v.2020). MetaD simulations, which utilize a biasing potential applied to one or multiple collective variables (or more simpler reaction coordinates), made use here of two reaction coordinates (RCs) to characterize the internal closed → open zipper-like conformational transition of C-terminal B-domain (or B-chain) residues. The RCs applied were the distances between Cα atoms of ValB12-TyrB26 (RC1) and GlyB8-ProB28 (RC2), which have been used previously in describing this core exposure (59, 60, 61). Simulation of insulin lispro (containing pairwise substitutions ProB28 → Lys and LysB29 → Pro) made use of the similar RCs, but with RC2 corresponding to GlyB8-LysB28. RCs were biased with Gaussians of initial height 0.5 kJ mol−1 and 0.05 Å width, applied every 2.5 ps. Rescaling of these Gaussians were made using a bias factor of 10, as was the case in well-tempered MetaD. To ensure sampling of relevant configurational space, restraining potentials were introduced to limit the maximum values of RC1 and RC2 to 45.0 Å with a force constant of 500 kJ mol−1. Likewise, restraints were applied to the backbone B-chain helix hydrogen bonds (between backbone carbonyl oxygen to amide nitrogen atoms) between residues SerB9-GlyB20, using a force constant of 100 kJ mol−1 and a distance of 3 Å. These restraints ensured that no artificial unfolding of the peptide core was induced which would sample exceedingly elongated RC distances, complicating the resultant free energy surface. The limit placed on the RC2 is well in excess of the distance of ≈27 Å observed for native insulin bound to hIR, and instead would represent a fully elongated B chain, parallel to the B-chain helix axis. In both WTMetaD and MWMetaD, two sets of each simulation were conducted both to 100 ns and 500 ns. For MWMetaD, 14 walkers were implemented. The free energy surfaces were calculated using the inbuilt PLUMED utility sum_hills and plotted as a two-dimensional surface as described below.
To determine simulation convergence, we made use of the block-average error-analysis technique, first reweighting the simulations to remove the effect of the biasing potential after discarding the first 100 ns of each walker (25% of the simulations). PLUMED block error-analysis scripts were utilized to calculate this convergence.
Generation of free energy surfaces
In exploring the effect of C-domain linker length on the ability of SCIs to engage the hIR, we investigated hydrophobic-core exposure of SCI analogs in comparison with HI. We employed the same RCs used in the MetaD simulations in all free energy surfaces (FESs; whether derived from conventional MD or MetaD simulations), which when projected along a two-dimensional FES demonstrate the occupancy of closed and open B-domain (or B-chain) hinge states. Such FESs therefore describe each of the hormones’ and analogs’ ability and proclivity to adopt a bound-like state. FESs were constructed using the Gromacs gmx distance and sham utilities and plotted using MATLAB (The MathWorks, Natick, MA).
Results
Interaction of SCI-b with IR-A site 1
The crystal structure of SCI-b in complex with the primary binding site of the hIR (μIR, comprising domains L1-CR [construct IR310.T] and an IR-A αCT derived peptide) and co-crystallized with an Fv 83-7 module (as a crystallization chaperone) was determined using diffraction data to resolution of 2.6 Å. Refinement statistics are presented in Table 2. The structure contains four copies of the complex in the crystallographic asymmetric unit (ASU). Very little intra-ASU variability was observed at the site of insulin:αCT engagement (0.7 Å average root-mean-square deviation [RMSD] across the backbone trace of the four insulin copies when respective domains L1 are overlaid [Fig. 2 A]). We hence used only a single ASU instance for purposes of comparison with the corresponding native insulin complex (PDB: 6VEP; determined using diffraction data to resolution 2.9 Å) (33); the overall mode of SCI-b-μIR binding is then seen to be similar to that of HI in complex with the same hIR receptor domains (Fig. 2 B). In the SCI-b complex, unambiguous electron density is observed for hIR domain L1-CR residues Gly5–Pro309 and all residues of the IR-A αCT peptide except its final residue Ser719. The SCI engages the L1-CR:αCT assembly via the now well-characterized detachment mechanism (18): residues PheB24 and PheB25 (within the aromatic triplet B24–B26) are observed detached from the peptide core and engage principally with αCT residues Phe714 and between Pro716 and Pro718, respectively. The electron density associated with the side chains of PheB24, TyrB16, Phe714, and Phe39 provides unambiguous rotameric detail of their packing at the core engagement sites, affirming the lack of complete shape complementarity (i.e., the presence of small cavities) in the vicinity of the PheB24 aromatic ring (33). Notably, TyrB16 is seen to engage L1-domain residue Phe39 via π-stacking. Interactions at the native interface, including GluA4 with Asn711, GlyB8 with His710, and ValB12 with His710, were likewise maintained in the SCI-b-μIR complex (Fig. 2 C).
Figure 2.
Crystal structures and comparison of SCI-b in complex with the μIR. (A) Superposition of the four monomers of SCI-b present within the asymmetric unit of the μIR crystal structure complex (PDB: 7KD6) compared with that of HI (PDB: 6VEP; white) also in complex with the μIR. TyrB26 through to C-domain residues C3–C5 (variable across ASU copies) were not resolved in the electron density maps of the SCI-B complex. (B) Structure of the SCI-b-bound μIR compared with that of HI bound to the same receptor construct (PDB: 6VEP; white). The IR CR domain is partly omitted for clarity. (C) Interaction between SCI-b and the IR αCT peptide (purple) and L1 domain (gray). (D) Interactions between residues B1–B7 and the remainder of SCI-b. The side chains of residues B1 to B7 and IleA13 as well as the backbone of residues forming disulfide bonds are shown in stick representation. Disulfide bonds are shown in yellow. (E) Electron density (2mFo-DFc; blue wireframe) associated with SCI-b residues B21–B25, C4–C6, and A1–A3 in the structure reported here (contour level = 1.0σ). In all panels, colors are as in Fig. 1A and C. To see this figure in color, go online.
As in the canonical structure of the free insulin T state (6), the amide nitrogen of LeuB6 forms an interchain hydrogen bond with the backbone carbonyl of CysA6, and PheB1 packs against IleA13 (Fig. 2 D); these T-state-specific features occur in all four copies of the ASU. Electron density for this B-chain N-terminal segment was not well defined in previous μIR structures. We caution, however, that these canonical features of free insulin may have been enforced here by crystal contacts, as the respective PheB1-LeuB6 segments border alternative ASU copies of the complex. As truncation of residues B1–B3 does not affect receptor binding (62), the above interactions are unlikely to be of physiological significance.
The present structure also permits characterization of several solvent molecules (unmodeled in all extant cryo-EM and crystal structures of the receptor ectodomain and its complexes). The discerned solvent molecules are mostly associated with the four Fv fragments that pack around the origin of the 222-point group symmetry, with a small number associated with the L1 domains. Surprisingly few solvent molecules appear to be associated with the SCI-b molecules, in contrast to the vast intermolecular hydrogen-bonding network observed previously in the free insulin dimer (6). No solvent molecule is discerned in the cavities surrounding the side chain of SCI-b PheB24.
Electron density corresponding to residues TyrB26-GlyC3 was not well resolved, echoing similar disorder across HI residues ThrB27-ThrB30 in the parent μIR complex (PDB: 6VEP; Fig. 2 B) and within parts of the C domain within homologous IGF complexes (Table 1) (33,63,64). B-domain residues beyond TyrB26 exhibit incomplete density, suggesting multiple conformations; analogs lacking these residues retain native receptor-binding affinity (as exemplified by des-tetrapeptide[B27-B30]-insulin and des-pentapeptide[B26-B30]-insulin-amide (65,66)). SCI-b C-domain residues are therefore likely to be mobile within the context of the μIR and without salient or persisting receptor interactions. C-domain residues ArgC5 and ArgC6, which are modeled tentatively, have varying main-chain dispositions across the four ASU copies. The overall location of unresolved C-domain residues must be within the volume between the domain L1, domain CR, and an adjacent μIR complex. Such variability may not occur in a holoreceptor complex due to the presence therein of adjacent receptor L2 domains. Despite limited or absent electron density for the B26-C6 residue segment, the inferred location of this segment with respect to αCT residues Val715–Ser719 is such that the latter αCT residues are threaded through the C-domain loop of the SCI (Fig. 2 E). Such topology mirrors recent cryo-EM and crystal structures of IGF-I (63,67) and IGF-II (64) bound to the IGF-1R, all of which have a threaded mode of growth factor binding with respect to αCT.
SCI-b contains stabilizing substitutions ThrA8 → His and TyrA14 → Glu (1,2). These side chains were not observed to form any specific intermolecular interactions in the context of the μIR. HisA8, incorporated both to optimize the A1–A8 helical C-terminal cap and augment receptor affinity (3), has been suggested to form a stabilizing interaction with the FnIII-1′ domain (33), not present in the μIR model. GluA14 is also directed toward solvent. The only additional SCI-b-specific substitutions (AspB28 and ProB29) lacked electron density, as discussed above.
Modeling SCI binding modes at IR-A site 1
Despite crystallographic deduction of αCT threading, the resultant locus of the C domain remained only partially resolved. We therefore employed MD simulations of both threaded and unthreaded binding modes to explore this issue further. We have previously explored models of SCI-b in complex with the μIR in which the C-terminal region of the A-isoform αCT was threaded through the C-domain and B-domain loop (B24–C6) (2). These prior simulations were extended here to include IR-B isoform-specific αCT in an effort to rationalize contrasting SCI-b affinity profiles between receptor isoforms (1).
Simulations of SCI-b bound to an L1-CR-L2 domain module with IR-A αCT peptide (Thr704–Ser719) maintained the classical insulin:hIR site-1 engagement motif. The SCI remained bound to the receptor fragments across five independent 1 μs simulations (Fig. 3 A). Structural features of the complex were reminiscent of the crystal structures of both HI and SCI-b bound to the μIR, with only minimal motion of the SCI with respect to the site-1 hormone-receptor interface observed (Fig. 3 B). Divergence was largely restricted to solvent-exposed moieties at the SCI chain termini (Fig. 3 C). Flexibility of B-chain N- and C-terminal regions in the simulations reflected the structural variation across the four copies in the crystallographic ASU. A notable deviation was at TyrB26, which adopted alternate rotameric orientations characterized by rotation around χ1 throughout the simulations. Alternate orientations of the TyrB26 side chain were nonetheless consistent with those observed in the structure of HI bound to the same domains (PDB: 6HN5). Although flexible, orientations were presumably facilitated by cation-π interactions of the B26 aromatic ring with the guanidinium groups of L1-domain residues Arg14 and Arg19 (Fig. 3 C). In both residues, the guanidinium groups engage the B26 phenol group in a planar “stacked” orientation. Likewise, AspB28 similarly forms transient side-chain contacts with Arg19, in addition to that of Arg270 and Arg271 via short-term salt bridges. Threading of the αCT peptide placed C-domain residues ArgC5 and ArgC6 near L2-domain residue Glu316, frequently forming a salt bridge (Fig. 3 D). Further variability was observed among solvent-exposed A-domain side chains of GluA4, GlnA5, and HisA8 (with respect to their respective positions in the SCI-b:hIR crystal structure).
Figure 3.
Molecular dynamics (MD) simulation of SCI-b and the IR L1-CR-L2 module with different αCT peptides. (A) Representative model of SCI-b bound to the hIR L1-CR-L2 module and an IR-A-derived αCT peptide. SCI-b is depicted in surface representation to highlight the threaded αCT peptide. (B) The root-mean-square deviation (RMSD) across backbone atoms of the SCI-b B-domain residues (residues B6–B24) that engage hIR domains L1 and αCT within the simulated complex in (A), against that of the experimentally determined structures of HI (PDB: 6VEP, top), and SCI-b (PDB: 7KD6, bottom) in complex with the same domains. The shaded regions represent the standard deviation, with the first 50 ns truncated for equilibration. (C) The root-mean-square fluctuation (RMSF) of SCI-b residues when simulated in complex with the IR L1-CR-L2 module and either an IR-A-derived αCT peptide (unthreaded) or IB-B-derived αCT peptide (both threaded and unthreaded). (D) Model of complexed SCI-b from simulation as presented in (A) show selected interactions between SCI-b and IR domain L2 as well as an intra-C-domain salt bridge. To see this figure in color, go online.
Simulations of SCI-b in complex with a threaded IR-B αCT peptide (Thr704–Ser731) broadly maintained the same conserved interactions observed in simulations of the shorter IR-A peptide and in the above co-crystal structure (Fig. 4 A). The threaded model recapitulated the αCT-mediated electrostatic interaction network involving the side chains of Arg717 and ArgB22 to GluA17 (present within both the structure of the complex between μIR and HI [PDB: 6VEP] and the complex with SCI-b presented here), and the C-terminal carboxylate of AsnA21 to Arg717 (Fig. 4 B). The 12-residue IR-B-specific insert (which lies two residues beyond the α-helical region of the αCT segment) remains unstructured, forming numerous nonspecific and short-lifetime interactions with both receptor domains L1 and L2 (Fig. 4 C). Engagement of the hormone by αCT is restricted to the region preceding the insert, with the side chain of PheB25 oriented beneath and between the side chains of Pro716 and Arg717; of IR-B insert residues, only one (Lys718) formed a sustained salt bridge to the C-domain acidic residues GluC1 and GluC2.
Figure 4.
MD simulations of SCI-b in complex with the IR L1-CR-L2 module and an IR-B isoform αCT peptide. (A) Surface representation of the complex. (B) Salient interactions between SCI-b and domain L2 and the IR-B αCT peptide. (C) The 12-residue IR-B insert is mobile throughout simulations and is shown in blue through white to red over a single trajectory obtained during simulation. (D) Structural representation whereby the IR-B αCT domain is not threaded through the hormone C-domain B-domain loop. The structure of HI in complex with the μIR (PDB: 6VEP) is overlaid in white, with black arrows to indicate the degree of disparity between binding modes (PDB: 6VEP domain L1 and the αCT domain removed for clarity). (E) Simulated model of SCI-b in complex with the L1-CR-L2 module with the IR-B αCT peptide partially threaded through the hormone C-domain B-domain loop. The inset details a top-down view with respect to the left. All models are colored as in Fig. 1A and C. To see this figure in color, go online.
MD simulations were then extended from the above IR-B αCT-threaded models to unthreaded models (Fig. 4 D) and partially threaded models (Fig. 4 E). Simulations that began from unthreaded IR-B αCT conformations exhibited significant departure from the archetypal site-1 insulin complex: displacement from the L1 interface led to partial dissociation over independent 1 μs trajectories (Fig. 4 D). SCI conformations that compacted the C domain against the α-helical core were needed to accommodate an unthreaded αCT, which in turn then perturbed the canonical site-1 interface, including the defining contacts made by residues PheB24 and PheB25. The C domain, unable to interact with the receptor owing to the intervening αCT, promoted SCI dissociation, similar to that previously observed in simulations of the μIR complex with an unthreaded IR-A αCT peptide (2). Models which instead forced the cis conformation of αCT Pro716 allowing for an abrupt β-turn of the IR-B insert and unthreading of the αCT domain likewise did not explore the classical site-1 engagement motif and saw analog dissociation (data not shown). Simulations did, however, display transitions to partially threaded conformations wherein only the Pro716–Thr719 segment inserts between the SCI C and B domains, with the remainder of αCT segment oriented antiparallel to the upstream αCT helix (Fig. 4 E). Such partial threading was largely preserved in the trajectory, despite incomplete engagement of residues B25 and B26 and without any persistent contact between the SCI C and L2 domains. Transient side-chain interactions between GluC1 and Lys718 were observed, with the side chain of the threaded Arg717 maintaining the interaction to the C-terminal carboxylate of AsnA21, as observed in the fully threaded αCT simulations. Exposed to the C domain by the antiparallel orientation of the unthreaded αCT, ArgC5 also maintained a consistent salt bridge to Glu726 throughout the simulation.
ITC studies of variant αCT peptide assemblies
The ITC-determined affinities of SCI-b for native and variant minireceptor complexes are presented in Table 3. SCI-b’s baseline affinity determined using IR485 and IR-A αCT peptide (KD = 20.1 ± 8.4 nM) was similar to that of HI (KD = 30.5 ± 5.4 nM). Using IR-B αCT peptide (IR residues 704–731) instead reduced SCI-b affinity by approximately fourfold (KD = 160.6 ± 6.5 nM) relative to HI (KD = 37.3 ± 5.3 nM). This isoform-specific reduction is consistent with previous competitive-displacement binding assays (1,2): whereas HI binds the IR-A and -B holoreceptor isoforms with essentially equal affinity (0.07 ± 0.1 and 0.08 ± 0.1 nM, respectively (1)), holoreceptor binding of SCI-b was isoform-selective (0.2 ± 0.1 nM [IR-A] and 1.0 ± 0.3 nM [IR-B]) (1). Thus, preference of SCI-b for IR-A is not dependent on site 2 nor any of the three FnIII domains. We note in passing that the binding of SCI-b or HI to the IR-A and IR-B minireceptors is entropically distinct: binding to the B isoform is entropically less favorable (TΔS = −45.6 ± 1.3 kJ mol−1) than binding to the A isoform (TΔS = −56.1 ± 1.7 kJ mol−1). This difference aligns with the difference in relative receptor affinities that may be associated with the association of αCT of IR-B upon (partial) threading through the SCI-b loop.
Table 3.
Isothermal titration calorimetry of insulins against IR485 with an αCT peptidea
| Insulin analog | αCT peptide | KD (nM) | ΔH (kJ mol−1) | TΔS (kJ mol−1) |
|---|---|---|---|---|
| HI | IR-A αCT704–719 | 30.5 ± 5.4 | -63.6 ± 9.6 | −20.5 ± 9.2 |
| IR-B αCT704–731 | 37.3 ± 5.3 | −58.3 ± 2.1 | −15.1 ± 2.1 | |
| SCI-b | IR-A αCT704–719 | 20.1 ± 8.4 | −90.0 ± 0.4 | −45.6 ± 1.3 |
| IR-B αCT704–731 | 160.6 ± 6.5 | −94.6 ± 1.7 | −56.1 ± 1.7 |
Data are from three technical replicates; errors are SEM. For further details, see materials and methods.
The C domain affects insulin dynamics and receptor engagement
We next investigated the effects of the SCI C domain on stochastic motions of the B-chain C-terminal segment with respect to hydrophobic-core exposure. The detachment of this segment is essential to facilitate site-1 binding (17), with the associated free energy change referred to previously as the “free energy of strain” on binding (60). To test whether this motion is essential to achieve an active ligand conformation (12,68), a tightly constrained SCI with a C-domain linker length of −1 (11) was simulated for comparison with SCI-b (C-domain linker length of 6; Fig. 1 B). [B29-A1]-HI reduces IR affinity by ≈1500-fold (68).
Enhanced sampling metadynamics (MetaD) was implemented as described by Macháčková et al. (60). MetaD exploits a biasing potential along specified collective variables to enhance sampling of rare events, such as exposure of insulin’s core in the present application (for review, see (69)).
Reaction coordinates 1 and 2 (RC1 and RC2) were the respective distances between the Cα atoms of ValB12 and TyrB26 (RC1) and between the Cα atoms of GlyB8 and AspB28 (ProB28 in simulation of insulin lispro) (RC2), which provided biased variables. Simulations of well-tempered MetaD simulations of 500 ns with 14 multiple-walker replicates (multiple-walker metadynamics—MWMetaD) (Fig. S1) produced FESs that differed from a previous similar analysis. The FES of native insulin demonstrates the expected pseudo-diagonal symmetry with increases at both RC1 and RC2. The sampled regions indicate broadly that the system explores a configurational space associated with a zipper-like opening of the hormone core similar to that described previously (61). The present surface nevertheless differs in part from that obtained when the simulation begins from an open state (60); in particular, short well-tempered MetaD simulations (<100 ns) did not exhibit diffusive dynamics of the biased RC (data not shown), typical of a high-energy unbiased degree of freedom restricting convergence. In the present analysis, by contrast, convergence of the MWMetaD simulation was achieved as indicated by biased RC and highlighted through block analysis of the average free energy error as a function of block length (Fig. S2), both of which suggest thorough reconstruction of the energetics of an unbiased configurational change.
To mitigate any adverse effects of the biasing potential, we focused on long-time-scale unbiased conventional MD, extended to 25 μs (five replicates with 5 μs per replicate) per analog. In all analogs we sampled multiple core-exposure events (Fig. S3), indicating considerable sampling of all states and the transitions among them—a necessity in obtaining accurate converged free energies. The FES of monomeric HI (Fig. 5 A) displays a broad global free energy minimum (FE minimum), consistent with the previously described solution structures of HI (PDB: 2KJJ—insulin lispro) and is representative of a global minimum structure that corresponds to a closed C-terminal B-chain state. As expected, the FES of insulin lispro is very similar (Fig. S4). Corresponding distances RC1 and RC2, as derived from the NMR structure (PDB: 2KJJ), are 6.6 ± 0.1 Å and 9.5 ± 0.6 Å. These baseline FESs also exhibit in each case a local FE minimum describing open conformations wherein the C-terminal B-chain segment (B24–B30) is disengaged from the core, packing instead against the A-chain α-helices centered at RC distances 13.2 and 21.0 Å for RC1 and RC2 respectively. This arrangement is akin to conformations of HI bound to the IR site 1 (respective RC1 and RC2 distances 15.9 ± 1.3 Å and 25.1 ± 1.2 Å, as observed from PDB: 6PXW, 6CE9, 6VEP, and 6SOF).
Figure 5.
Analysis of monomeric insulin analogs from cumulative MD simulations. (A) Free energy surface (FES) of HI using the distances between Cα atoms of ValB12 and TyrB26 (RC1) and GlyB8 and ProB28 (RC2) as reaction coordinates. (B) FES of [B29-A1]-HI using the same RC as in (A). (C) FES of SCI-b using the same RC1 as in (A), and the distance between Cα atoms of GlyB8 and LysB28 (RC2). In all FESs, the dashed white oval represents the extent of RC distances of bound HI observed experimentally (from PDB: 6PXW, 6CE9, 6VEP, and 6SOF). The black rectangle in (A) represents the location of the free energy minimum of HI in a core-exposed state, with this same RC position indicated as a dashed black rectangle in (B) and (C). (D) Solvent-accessible surface area (SASA) of B-domain residues 21–30 from combined simulations of HI. (E) SASA of the same residues as in (D) from simulations of [B29-A1]-HI. (F) SASA of the same residues as in (D) from simulations of SCI-b. (G) Average helicity of A-chain residues of SCI-b (red) and [B29-A1]-HI (green) compared with HI (blue). The average helicity is determined individually for each residue from all five simulation replicas using the gmx helix utility within Gromacs (3); error bars depict standard deviation across replicates. To see this figure in color, go online.
The FE minimum of HI simulated in solution exhibits shorter RC distances relative to the receptor-bound conformation, with a corresponding free energy of strain (characterized by the free energy difference between closed- and hIR-bound configurations) difference of ≈10 kJ mol−1. The difference between the global closed and partial core-exposed observed minima is ≈4.0 kJ mol−1, with the lowest energy barrier of transition between states being ≈8.6 kJ mol−1. The closed state is promoted by nonpolar interactions among the side chains of PheB24, TyrB26, IleA2, ValA3, and TyrA19. The unzippered exposure of the B-chain C-terminal segment is initiated by movement of the side chain of PheB25, with a hydrogen bond maintained between the amide nitrogen of AsnA21 and carbonyl oxygen of GlyB23 as a β-turn anchor. A T-state-specific hydrogen bond between the PheB25 amide and TyrA19 carbonyl dissociates with rotation of the TyrA19 phenol ring around χ1. The latter conformation would orient the TyrA19 para-hydroxyl group toward PheB24 (70).
[B29-A1]-HI is a more tightly constrained modified insulin containing bifunctional reagents inserted between the ε-amino group of LysB29 and α-amino group of GlyA1 (12). Simulation of [B29-A1]-HI uncovered significant restriction in the RC conformational space relative to HI (Fig. 5 B). Its FES exhibited only a single, relatively constrained FE minimum, which corresponded to an analogous closed state as in HI. Nonetheless, the stochastic motions within the B-chain C-terminal segment enabled partial exposure of the hormone core, as indicated by exploration of phase space with increased RC distances, albeit to an extent significantly reduced relative to HI.
The SCI-b FES exhibits a single, broad FE minimum corresponding to a closed B domain (Fig. 5 C). This minimum is centered around 7.5 Å (RC1) and 8.0 Å (RC2), similar to the distances in its solution structure (PDB: 5WBT) with RC1 and RC2 distances of 7.0 ± 0.1 Å and 8.9 ± 1.0 Å, respectively. A slight reduction was observed in the extent of conformational space explored relative to HI. As in previous simulations of SCI-b (2), GluC1 and GluC2 frequently form salt bridges to ArgC5 and ArgC6. Alternative salt bridges occur between the latter basic side chains and flanking acidic residues GluA4 and AspB28. No electrostatic interactions were observed between the C domain and central B-domain α-helix that could otherwise have limited core exposure. Despite an absence of an open FE minimum (as seen in HI simulations; Fig. 5 C, dashed rectangle), SCI-b sampled partially open conformations with an FE penalty of ≈9 kJ mol−1, but only partially sampled the receptor-bound conformation (Fig. 5 C, dashed white oval). These findings suggest that initial binding of SCI-b to hIR or its fragments is integral to further opening of the hormone core.
Within the analogs simulated, C-terminal B-domain detachment (residues B21–B30) is broadly consistent with an increase in solvent-accessible surface area (SASA; Fig. 5, D–F). The mean overall SASAs of HI ranks between that of [B29-A1]-HI and SCI-b (3953 Å2 for HI, 3807 and 4361 Å2 for [B29-A1]-HI and SCI-b, respectively). As indicated by respective FESs, the SCI-b C domain reduces B-domain detachment; hydration of B-domain residues is likewise reduced. Furthermore, average helicity within the A chain is lower in [B29-A1]-HI than in SCI-b, especially within the N-terminal A-chain α-helix (residues A1–A8). Although there is significant fluctuation in helicity in both (Fig. 5 G), [B29-A1]-HI demonstrates greater variability in secondary structure, suggesting an impact of the shorter linker.
Very short C domains are not compatible with IR engagement
Finally, although the above may rationalize the very low hIR affinity of [B29-A1]-HI (12), we also undertook MD simulations of site-1 complexes containing this analog (Fig. S5). Models of the [B29-A1]-HI minireceptor complex (assessed over five replicas for 1 μs each, totaling 5 μs duration) were broadly unstable. Whereas conformational variation was observed across replicates, none recapitulated the native receptor-binding surface spanning the N-terminal A-chain α-helix and B-chain “aromatic triplet” (Fig. S5). The canonical contacts between αCT residue Phe714 and this surface (comprising the side chains of IleA2, LeuB11, ValB12, LeuB15, and PheB24) were rarely present. The B29-A1 constraint distorted the N-terminal A-chain α-helix in two of the five replicates and altered core packing in all five replicates. Although such perturbations may in principle aid hormone dissociation (2), this did not occur, presumably due to anchoring of the analog by the threaded αCT peptide (imposed in the starting models).
Discussion
The single-chain topology of SCIs delays or prevents the cross-β assembly of insulin fibrils at or above room temperature (71,72) and so promises a therapeutic platform circumventing the difficulties present in the current global cold chain (4,5). In the present study, we sought to visualize how an SCI binds to IR site 1, the primary hormone-binding site (17), an event proposed to trigger large-scale receptor reorganization (20, 21, 22, 23, 24). Despite the C domain of SCI-b being only partially defined in our μIR co-crystal structure, density assigned to C-domain residues ArgC5 and ArgC6 (Fig. 2) restricts the space available for preceding C-domain residues with respect to the visible C-terminal region of the αCT peptide. This restriction leads us to deduce a threaded mode of binding within the crystallized μIR complex.
The threaded arrangement was supported by MD simulations of the complex over multiple independent replicas. In such simulations, SCI-b exhibits robust stability of the interaction with an L1-CR-L2 domain module only when the αCT domain is threaded (Fig. 3). Simulations of an unthreaded αCT peptide demonstrated structural instability at its interface with L1:αCT (Fig. 4), leading to its eventual dissociation. Despite this instability, the unthreaded and partially threaded models suggest a possible pathway to the final threaded state. The partially threaded intermediate state could in principle facilitate full threading of the C-terminal region of the αCT domain in a reverse direction, whereby the N-terminal portion of the unstructured αCT segment is first threaded (Fig. S6). Such a mechanism would require the C-domain linker to be sufficiently long to allow αCT to thread the loop twice (in the forward and reverse directions), thus placing an additional constraint on SCIs that bind with threading of the IR-B insert. The C domain of an SCI would thus exhibit a threshold length below which threading the insert would be disfavored or precluded. This restriction would not hinder site-2 binding, whereby hormone core exposure is not observed (20, 21, 22, 23, 24). Shortened SCI analogs are able to elicit activity seemingly via site 2 exclusively at high concentration (68).
The MD simulations further demonstrate both 1) an absence of secondary structure in the IR-B insert segment (as previously predicted (28)) and 2) significant motion associated with the entire C-terminal region of the insert. We note that in apo-IGF-1R (63), the C-terminal region of its αCT segment forms (and is stabilized by) electrostatic interactions. Despite IGF-1R αCT being similar in length to the shorter IR-A isoform-specific αCT (73), IR receptor domains may similarly stabilize the IR-B insert in its apo form. This might facilitate engagement with two-chain insulin analogs and enable threading of SCIs by acting as a conformational restraint, i.e., enabling a forward-threading mechanism whereby the C-terminal end of αCT leads.
For the insulin analogs tested here, dissociation constants determined using IR-A-derived αCT peptides were consistent with previous studies (33,48,74). In contrast, SCI-b displayed a salient difference in binding IR485 in co-complex with an IR-B-derived αCT peptide relative to assays employing the IR holoreceptor (1). This difference suggests that the affinity of SCI-b to the hIR is in part facilitated by receptor domains not present in the domain-minimized IR485 construct, possibly via site-2 interactions or IR-B insert stabilization. Even as the binding mode of SCI-b to IR-B is entropically distinct to that to IR-A, the significance of these observations is uncertain. Whereas determination of free energies (ΔG) via ITC is both robust and accurate, individual inferred changes in enthalpy (ΔH) and entropy (TΔS) are less so (75). Understanding the location of IR-B insert residues in both the hormone-bound and unbound states may clarify the origins of both isoform selectivity, and the difference in kinetics among insulin analogs from the alternate receptor isoforms (76, 77, 78). Notably, despite the limited and contrasting affinity and kinetic data available within the literature of IR-A versus IR-B, it is evident there is a difference between the kon and koff rates of HI between isoforms (79,80), estimated to be a threefold increased association and twofold increased dissociation at the IR-A versus IR-B isoform. Threading of the longer IR-B αCT in SCIs would presumably further result in decreased association and dissociation rates.
Stochastic exposure of the SCI B-chain C-terminal region was simulated in an effort to explore the ability of SCIs to engage the hIR at site 1. Whereas initial studies making use of “mini-proinsulins” with connecting C domains of fewer than three residues exhibited poor potency (less than 5% that of HI) (12,81), C domains containing more than three residues exhibited only a modest decrease in potency (>40% that of HI) (73,82,83). The relationship between the propensity of an insulin analog to adopt an open C-terminal B-chain conformation and receptor affinity has been explored previously via a combination of experimental and computational techniques (61,84), with increases in potency correlating with a reduction in the thermodynamic stability of the free state (85).
In assessing B-chain exposure, we were unable to replicate the insulin core-exposure FES using a previous MetaD technique, and we hence made use instead of multiple-walker MetaD simulations (Fig. S1). Variability is notable in descriptions of simulated HI FE minima among studies (60,61), highlighting the need to ensure sufficient sampling and robust, explicit error analysis. To this end, we opted to complement MetaD simulations with multiple replicas of conventional non-biased MD over extended simulation durations, markedly greater than employed previously and hence providing a more robust estimate of core-exposure free energies within the limits of the implemented simulation time. Importantly, the FES of HI from both MetaD and conventional MD simulations presented here agreed in both the features of the landscape and the respective free energies of each sampled state (Figs. S1 and 5 A).
The present FES simulations of SCIs demonstrate that SCIs with very short C domains (such as in [B29-A1]-HI) do not retain notable affinity for IR site 1 (Figs. 5 and S5). The FES depicts a significantly reduced propensity for C-terminal B-domain detachment, a reduction in configurational phase space exploration, and thereby limited exposure of key core residues: the simulations did not explore configurational space associated with an hIR-bound conformation, and therefore the free energy of strain is likely in excess of 16 kJ mol−1. [B29-A1]-HI’s low propensity (or impaired capacity) to adopt an active binding conformation is in accordance with the instability at the hIR site-1 interface. Other SCI analogs with foreshortened C domains (of two residues) B(1–30)-AK-A(1–21) (82) or B(1–29)-AAK-A(1–21) (86) also demonstrate impaired affinities: 0.14% and 0.5%, respectively, relative to HI. Furthermore, substitution of ThrB30 by Ala abated this effect by ≈3.5-fold (as in B(1–29)-AAK-A(1–21)); directed optimization as carried out for SCI-b may thus enable near-native affinity to be restored for somewhat shorter linker analogs.
Although detachment of the B-chain C-terminal region (B23–B30) is associated with potency and hIR affinity as in past simulations by other laboratories (87), we note that this correlation is not uniform. As a seeming paradox, substitution of ArgB22 → Gln in the B20–B23 β-turn was found to impair binding to IR-A by fivefold relative to HI as it distorts the turn which facilitates opening of the B chain (88). Conversely, GlyB24-insulin (a mutation of the key aromatic triplet residue PheB24) exhibits native-like affinity while destabilizing the B20–B30 segment (PDB: 1HIT) (89). Such retained affinity has been ascribed to a “register shift” at the hormone-receptor interface whereby PheB25 docks in the B24-binding pocket and TyrB26 plays the role of PheB25 in the native interface (90,91). These examples suggest that the propensity for opening the B chain is not in itself predictive of engaging the receptor and subsequently tight affinity: details of the variant interface play a greater role. That SCI-b and HI exhibit similar receptor affinities for the IR-A isoform hIR—despite differences in opening probability—presumably reflects compensation among multiple biophysical contributions to binding.
Conclusions
One year on from the centennial of insulin’s clinical introduction (92), the hormone remains a landmark in molecular medicine (93). The susceptibility of the hormone to degradation above room temperature (94) has imposed the necessity of a complex and costly cold chain of shipment, distribution, and storage (4,5). Given the growing pandemic of type 2 diabetes mellitus (95) and increasing global incidence of type 1 diabetes mellitus (96), SCIs are of potential therapeutic interest as an ultrastable platform for which a cold chain would be unnecessary (71,97). This frontier of “ultrabasal” once-a-week SCI analogs (combined with fusion proteins for prolonged circulation) is currently being explored (98). Understanding and tailoring SCI analogs with threading the αCT domain in mind is essential in facilitating these new therapeutics. This prospect has motivated our interest in structural principles governing SCI-receptor interactions.
The present crystal structure has exploited a domain-minimized model of the receptor’s primary hormone-binding site (17). The structure provides evidence for a threaded mode of αCT binding in a site-1 μIR-SCI complex. Simulations reinforce this finding and suggest a possible mechanism for threading whereby the first few residues of the C-terminus of the B chain initially partially “open,” followed by reverse threading of the remainder of the peptide.
Our study is limited by the use of the μIR construct rather than the intact ectodomain or holoreceptor. Although this domain-minimized model facilitates crystallization, it omits site 2 and leaves undefined the extent to which allosteric reorganization of the SCI-ectodomain complex recapitulates that triggered on binding of native insulin. As a technical issue, some aspects of the present structure (particularly those involving the N-terminal segment of the B domain) might reflect in part crystal contacts. Comparison of the detailed conformation of the C domain with homologous structures was limited by the absence of structures in which the positions of all C-domain residues are well defined. In the future, these limitations may be addressed through cryo-EM studies of the intact ectodomain (19,22,24) or holoreceptor (20,21,23).
Therapeutic applications of SCIs may transcend stability and shelf life. Of key future interest, for example, will be understanding potential mechanisms of isoform-specific IR binding by an SCI. We imagine that IR isoform selectivity may be engineered via C-domain modifications. This strategy could in principle exploit hormone-receptor contacts absent in native insulin-IR complexes. This class of analogs (29) could be of therapeutic interest in relation to biased post-receptor signaling (99,100), an important frontier of insulin pharmacology in type 2 diabetes mellitus (101).
Author contributions
N.A.S. carried out all simulations and analyzed the data. J.G.M. carried out protein expression, protein crystallization, and x-ray diffraction analysis. N.A.S. and J.G.M. carried out ITC studies. M.A.W. designed the SCI analogs and oversaw their purification. M.C.L. conducted x-ray diffraction analysis. N.A.S. wrote the initial article. All authors contributed to data interpretation and review of the article. M.A.W., M.C.L., and B.J.S. designed and directed the research.
Data availability
The coordinates of the structures determined here and their associated structure factors have been deposited in the Protein Data Bank (accession code PDB: 7KD6). Molecular dynamics and modeling data supporting this work can be accessed from the La Trobe University FigShare repository: https://opal.latrobe.edu.au/projects/Single-chain_Insulin_Analogs_Threaded_by_the_Insulin_Receptor_CT_Domain/147667.
Acknowledgments
This work is supported by NHMRC project grant number APP1143546 to M.C.L. M.C.L.’s research is also made possible at WEHI through Victorian State Government Operational Infrastructure Support and the Australian NHMRC Independent Research Institutes Infrastructure Support Scheme. Part of this work was undertaken using resources from the National Computational Infrastructure, which is supported by the Australian Government and provided through Intersect Australia, enabled by LIEF grant LE170100032, and through the HPC-GPGPU facility, which was established with the assistance of LIEF grant LE170100200. Research at the Indiana University School of Medicine was supported in part by grants from the National Institutes of Health (R01 DK127721 and R01 DK124401 to M.A.W.). The authors thank Dr. Ruitao Jin (La Trobe University) for helpful discussions, Dr. M.D. Glidden (Case Western Reserve University) and Dr. Y. Lu (Thermalin) for SCI expression and purification, and Ms. M. Margetts (WEHI) for production of the heavy- and light-chain fragments of Fv 83-7.
Declaration of interests
M.A.W. is a co-founder of Thermalin, Inc. for which he has served as Chief Innovation Officer, has consulted, and holds stock.
Editor: Alan Grossfield
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.09.038.
Contributor Information
Michael A. Weiss, Email: weissma@iu.edu.
Michael C. Lawrence, Email: lawrence@wehi.edu.au.
Brian J. Smith, Email: brian.smith@latrobe.edu.au.
Supporting material
References
- 1.Glidden M.D., Aldabbagh K., et al. Weiss M.A. An ultra-stable single-chain insulin analog resists thermal inactivation and exhibits biological signaling duration equivalent to the native protein. J. Biol. Chem. 2018;293:47–68. doi: 10.1074/jbc.M117.808626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glidden M.D., Yang Y., et al. Weiss M.A. Solution structure of an ultra-stable single-chain insulin analog connects protein dynamics to a novel mechanism of receptor binding. J. Biol. Chem. 2018;293:69–88. doi: 10.1074/jbc.M117.808667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hua Q.x., Nakagawa S.H., et al. Weiss M.A. Design of an active ultrastable single-chain insulin analog: synthesis, structure, and therapeutic implications. J. Biol. Chem. 2008;283:14703–14716. doi: 10.1074/jbc.M800313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemann L., Braune K., et al. Krämer L.A. Insulin storage: a critical reappraisal. J. Diabetes Sci. Technol. 2021;15:147–159. doi: 10.1177/1932296819900258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maikawa C.L., Mann J.L., et al. Appel E.A. Engineering insulin cold chain resilience to improve global access. Biomacromolecules. 2021;22:3386–3395. doi: 10.1021/acs.biomac.1c00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker E.N., Blundell T.L., et al. Reynolds C.D. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1988;319:369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- 7.Dodson G., Steiner D. The role of assembly in insulin’s biosynthesis. Curr. Opin. Struct. Biol. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Hua Q.X., et al. Weiss M.A. Solution structure of proinsulin: connecting domain flexibility and prohormone processing. J. Biol. Chem. 2010;285:7847–7851. doi: 10.1074/jbc.C109.084921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams M.J., Blundell T.L., et al. Sheat S. Structure of rhombohedral 2 zinc insulin crystals. Nature. 1969;224:491–495. [Google Scholar]
- 10.Jin Chan S., Steiner D.F. Insulin through the ages: phylogeny of a growth promoting and metabolic regulatory hormone. Am. Zool. 2000;40:213–222. [Google Scholar]
- 11.Nakagawa S.H., Tager H.S. Perturbation of insulin-receptor interactions by intramolecular hormone cross-linking. Analysis of relative movement among residues A1, B1, and B29. J. Biol. Chem. 1989;264:272–279. [PubMed] [Google Scholar]
- 12.Derewenda U., Derewenda Z., et al. Markussen J. X-ray analysis of the single chain B29-A1 peptide-linked insulin molecule. J. Mol. Biol. 1991;220:425–433. doi: 10.1016/0022-2836(91)90022-x. [DOI] [PubMed] [Google Scholar]
- 13.Cutfield J., Cutfield S., et al. Dodson E. Evidence concerning insulin activity from the structure of a cross-linked Derivative. Hoppe. Seylers. Z. Physiol. Chem. 1981;362:755–761. doi: 10.1515/bchm2.1981.362.1.755. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence M.C. Understanding insulin and its receptor from their three-dimensional structures. Mol. Metabol. 2021;52:101255. doi: 10.1016/j.molmet.2021.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turvey S.J., McPhillie M.J., et al. Fishwick C.W.G. Recent developments in the structural characterisation of the IR and IGF1R: implications for the design of IR–IGF1R hybrid receptor modulators. RSC Med. Chem. 2022;13:360–374. doi: 10.1039/d1md00300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKern N.M., Lawrence M.C., et al. Ward C.W. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature. 2006;443:218–221. doi: 10.1038/nature05106. [DOI] [PubMed] [Google Scholar]
- 17.Menting J.G., Whittaker J., et al. Lawrence M.C. How insulin engages its primary binding site on the insulin receptor. Nature. 2013;493:241–245. doi: 10.1038/nature11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menting J.G., Yang Y., et al. Lawrence M.C. Protective hinge in insulin opens to enable its receptor engagement. Proc. Natl. Acad. Sci. USA. 2014;111:E3395–E3404. doi: 10.1073/pnas.1412897111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weis F., Menting J.G., et al. Lawrence M.C. The signalling conformation of the insulin receptor ectodomain. Nat. Commun. 2018;9:4420. doi: 10.1038/s41467-018-06826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J., Park J., et al. Bai X.C. Synergistic activation of the insulin receptor via two distinct sites. Nat. Struct. Mol. Biol. 2022;29:357–368. doi: 10.1038/s41594-022-00750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen J., Brandt J., et al. Nissen P. Structural investigations of full-length insulin receptor dynamics and signalling. J. Mol. Biol. 2022;434:167458. doi: 10.1016/j.jmb.2022.167458. [DOI] [PubMed] [Google Scholar]
- 22.Gutmann T., Schäfer I.B., et al. Coskun Ü. Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain. J. Cell Biol. 2020;219:e201907210. doi: 10.1083/jcb.201907210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchikawa E., Choi E., et al. Bai X.-C. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor–ligand complex. Elife. 2019;8:e48630. doi: 10.7554/eLife.48630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scapin G., Dandey V.P., et al. Carragher B. Structure of the insulin receptor-insulin complex by single-particle cryo-EM analysis. Nature. 2018;556:122–125. doi: 10.1038/nature26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Meyts P., Roth J., et al. Lesniak M.A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem. Biophys. Res. Commun. 1973;55:154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]
- 26.De Meyts P. The structural basis of insulin and insulin-like growth factor-I receptor binding and negative co-operativity, and its relevance to mitogenic versus metabolic signalling. Diabetologia. 1994;37:S135–S148. doi: 10.1007/BF00400837. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y.-S., Gleaton J., et al. Weiss M.A. Insertion of a synthetic switch into insulin provides metabolite-dependent regulation of hormone–receptor activation. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2103518118. e2103518118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menting J.G., Lawrence C.F., et al. Lawrence M.C. Structural congruency of ligand binding to the insulin and insulin/type 1 insulin-like growth factor hybrid receptors. Structure. 2015;23:1271–1282. doi: 10.1016/j.str.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Jiráček J., Žáková L. Structural perspectives of insulin receptor isoform-selective insulin analogs. Front. Endocrinol. 2017;8:167. doi: 10.3389/fendo.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y., Margetts M.B., et al. Lawrence M.C. How insulin-like growth factor I binds to a hybrid insulin receptor type 1 insulin-like growth factor receptor. Structure. 2022;30:1098–1108.e6. doi: 10.1016/j.str.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belfiore A., Malaguarnera R., et al. Vigneri R. Insulin receptor isoforms in physiology and Disease: an updated view. Endocr. Rev. 2017;38:379–431. doi: 10.1210/er.2017-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soos M.A., Siddle K., et al. Lennox E.S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem. J. 1986;235:199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong X., Menting J.G., et al. Chou D.H.C. A structurally minimized yet fully active insulin based on cone-snail venom insulin principles. Nat. Struct. Mol. Biol. 2020;27:615–624. doi: 10.1038/s41594-020-0430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aragão D., Aishima J., et al. Caradoc-Davies T.T. MX2: a high-flux undulator microfocus beamline serving both the chemical and macromolecular crystallography communities at the Australian Synchrotron. J. Synchrotron Radiat. 2018;25:885–891. doi: 10.1107/S1600577518003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabsch W. Xds. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karplus P.A., Diederichs K. Linking crystallographic model and data quality. Science. 2012;336:1030–1033. doi: 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCoy A.J., Grosse-Kunstleve R.W., et al. Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams P.D., Afonine P.V., et al. Zwart P.H. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emsley P., Lohkamp B., et al. Cowtan K. Features and development of coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bricogne G., Blanc E., et al. Vonrhein C. 2017. BUSTER. [Google Scholar]
- 41.Winn M.D., Isupov M.N., Murshudov G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 42.Smart O.S., Womack T.O., et al. Bricogne G. Exploiting structure similarity in refinement: automated NCS and target-structure restraints in BUSTER. Acta Crystallogr. D Biol. Crystallogr. 2012;68:368–380. doi: 10.1107/S0907444911056058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou M., Garrett T.P.J., et al. Ward C.W. The first three domains of the insulin receptor differ structurally from the insulin-like growth factor 1 receptor in the regions governing ligand specificity. Proc. Natl. Acad. Sci. USA. 2006;103:12429–12434. doi: 10.1073/pnas.0605395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celikel R., Ruggeri Z.M., Varughese K.I. von Willebrand factor conformation and adhesive function is modulated by an internalized water molecule. Nat. Struct. Biol. 2000;7:881–884. doi: 10.1038/79639. [DOI] [PubMed] [Google Scholar]
- 45.Berry M.B., Johnson K.A., et al. Phillips G.N. Structure of an anti-HIV monoclonal Fab antibody fragment specific to a gp120 C-4 region peptide. Proteins. 2001;45:281–282. doi: 10.1002/prot.1148. [DOI] [PubMed] [Google Scholar]
- 46.Teplyakov A., Obmolova G., et al. Gilliland G.L. On the domain pairing in chimeric antibodies. Mol. Immunol. 2010;47:2422–2426. doi: 10.1016/j.molimm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Williams C.J., Headd J.J., et al. Richardson D.C. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 2018;27:293–315. doi: 10.1002/pro.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menting J.G., Ward C.W., et al. Lawrence M.C. A thermodynamic study of ligand binding to the first three domains of the human insulin receptor: relationship between the receptor α-chain C-terminal peptide and the site 1 insulin mimetic peptides. Biochemistry. 2009;48:5492–5500. doi: 10.1021/bi900261q. [DOI] [PubMed] [Google Scholar]
- 49.Keller S., Vargas C., et al. Schuck P. High-precision isothermal titration calorimetry with automated peak-shape analysis. Anal. Chem. 2012;84:5066–5073. doi: 10.1021/ac3007522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houtman J.C.D., Brown P.H., et al. Schuck P. Studying multisite binary and ternary protein interactions by global analysis of isothermal titration calorimetry data in SEDPHAT: application to adaptor protein complexes in cell signaling. Protein Sci. 2007;16:30–42. doi: 10.1110/ps.062558507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics. 2016;54:5.6.1–5.6.37. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparrow L.G., Lawrence M.C., et al. Ward C.W. N-linked glycans of the human insulin receptor and their distribution over the crystal structure. Proteins. 2008;71:426–439. doi: 10.1002/prot.21768. [DOI] [PubMed] [Google Scholar]
- 53.Abraham M.J., Murtola T., et al. Lindahl E. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 54.Guvench O., Mallajosyula S.S., et al. MacKerell A.D. CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J. Chem. Theor. Comput. 2011;7:3162–3180. doi: 10.1021/ct200328p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Best R.B., Zhu X., et al. MacKerell A.D. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ1 and χ2 Dihedral angles. J. Chem. Theor. Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parrinello M., Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 1981;52:7182–7190. [Google Scholar]
- 57.Hess B. P-LINCS: a parallel linear constraint solver for molecular simulation. J. Chem. Theor. Comput. 2008;4:116–122. doi: 10.1021/ct700200b. [DOI] [PubMed] [Google Scholar]
- 58.Tribello G.A., Bonomi M., et al. Bussi G. Plumed 2: new feathers for an old bird. Comput. Phys. Commun. 2014;185:604–613. [Google Scholar]
- 59.Žáková L., Kletvíková E., et al. Brzozowski A.M. Structural integrity of the B24 site in human insulin is important for hormone functionality. J. Biol. Chem. 2013;288:10230–10240. doi: 10.1074/jbc.M112.448050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macháčková K., Mlčochová K., et al. Jiráček J. Mutations at hypothetical binding site 2 in insulin and insulin-like growth factors 1 and 2 result in receptor- and hormone-specific responses. J. Biol. Chem. 2019;294:17371–17382. doi: 10.1074/jbc.RA119.010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papaioannou A., Kuyucak S., Kuncic Z. Molecular dynamics simulations of insulin: elucidating the conformational changes that enable its binding. PLoS One. 2015;10:e0144058. doi: 10.1371/journal.pone.0144058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerp L., Steinhilber S., et al. Geiger R. Changes in immunospecificity and biologic activity of bovine insulin due to subsequent removal of the amino acids B1, B2 and B3. Diabetes. 1974;23:651–656. doi: 10.2337/diab.23.8.651. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y., Kong G.K.-W., et al. Lawrence M.C. How ligand binds to the type 1 insulin-like growth factor receptor. Nat. Commun. 2018;9:821. doi: 10.1038/s41467-018-03219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y., Kirk N.S., et al. Lawrence M.C. How IGF-II binds to the human type 1 insulin-like growth factor receptor. Structure. 2020;28:786–798.e6. doi: 10.1016/j.str.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer W.H., Saunders D., et al. Zahn H. A shortened insulin with full in vitro potency. Biol. Chem. Hoppe Seyler. 1985;366:521–525. doi: 10.1515/bchm3.1985.366.1.521. [DOI] [PubMed] [Google Scholar]
- 66.Záková L., Barth T., et al. Zórad S. Shortened insulin analogues: marked changes in biological activity resulting from replacement of TyrB26 and N-methylation of peptide bonds in the C-terminus of the B-chain. Biochemistry. 2004;43:2323–2331. doi: 10.1021/bi036001w. [DOI] [PubMed] [Google Scholar]
- 67.Li J., Choi E., et al. Bai X.C. Structural basis of the activation of type 1 insulin-like growth factor receptor. Nat. Commun. 2019;10:4567. doi: 10.1038/s41467-019-12564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hua Q.X., Hu S.Q., et al. Weiss M.A. Mini-proinsulin and Mini-IGF-I: homologous protein sequences encoding non-homologous structures. J. Mol. Biol. 1998;277:103–118. doi: 10.1006/jmbi.1997.1574. [DOI] [PubMed] [Google Scholar]
- 69.Bussi G., Laio A. Using metadynamics to explore complex free-energy landscapes. Nat. Rev. Phys. 2020;2:200–212. [Google Scholar]
- 70.Jacoby E., Hua Q.X., et al. Weiss M.A. Structure and dynamics of a protein assembly. 1H-NMR studies of the 36 kDa R6 insulin hexamer. J. Mol. Biol. 1996;258:136–157. doi: 10.1006/jmbi.1996.0239. [DOI] [PubMed] [Google Scholar]
- 71.Phillips N.B., Whittaker J., et al. Weiss M.A. Insulin fibrillation and protein design: topological resistance of single-chain analogs to thermal degradation with application to a pump reservoir. J. Diabetes Sci. Technol. 2012;6:277–288. doi: 10.1177/193229681200600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hua Q.X., Weiss M.A. Mechanism of insulin fibrillation: the structure of insulin under amyloidogenic conditions resembles a protein-folding intermediate. J. Biol. Chem. 2004;279:21449–21460. doi: 10.1074/jbc.M314141200. [DOI] [PubMed] [Google Scholar]
- 73.Kristensen C., Andersen A.S., et al. Kjeldsen T. A single-chain insulin-like growth factor I/insulin hybrid binds with high affinity to the insulin receptor. Biochem. J. 1995;305:981–986. doi: 10.1042/bj3050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiong X., Blakely A., et al. Chou D.H.C. Symmetric and asymmetric receptor conformation continuum induced by a new insulin. Nat. Chem. Biol. 2022;18:511–519. doi: 10.1038/s41589-022-00981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chodera J.D., Mobley D.L. Entropy-enthalpy compensation: role and ramifications in biomolecular ligand recognition and design. Annu. Rev. Biophys. 2013;42:121–142. doi: 10.1146/annurev-biophys-083012-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Páníková T., Mitrová K., et al. Jiráček J. Insulin analogues with altered insulin receptor isoform binding specificities and enhanced aggregation stabilities. J. Med. Chem. 2021;64:14848–14859. doi: 10.1021/acs.jmedchem.1c01388. [DOI] [PubMed] [Google Scholar]
- 77.Glendorf T., Stidsen C.E., et al. Kjeldsen T. Engineering of insulin receptor isoform-selective insulin analogues. PLoS One. 2011;6:e20288. doi: 10.1371/journal.pone.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vienberg S.G., Bouman S.D., et al. Nishimura E. Receptor-isoform-selective insulin analogues give tissue-preferential effects. Biochem. J. 2011;440:301–308. doi: 10.1042/BJ20110880. [DOI] [PubMed] [Google Scholar]
- 79.Knudsen L., De Meyts P., Kiselyov V.V. Insight into the molecular basis for the kinetic differences between the two insulin receptor isoforms. Biochem. J. 2011;440:397–403. doi: 10.1042/BJ20110550. [DOI] [PubMed] [Google Scholar]
- 80.Goyal P., Pai H.V., et al. Ullanat R. Physicochemical and functional characterization of MYL-1501D, a proposed biosimilar to insulin glargine. PLoS One. 2021;16:e0253168. doi: 10.1371/journal.pone.0253168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Markussen J., Jørgensen K.H., et al. Thim L. Single chain des-(B30) insulin: intramolecular crosslinking of insulin by trypsin catalyzed transpeptidation. Int. J. Pept. Protein Res. 1985;26:70–77. [PubMed] [Google Scholar]
- 82.Huang Y., Liang Z., Feng Y. The relationship between the connecting peptide of recombined single chain insulin and its biological function. Sci. China C Life Sci. 2001;44:593–600. doi: 10.1007/BF02879353. [DOI] [PubMed] [Google Scholar]
- 83.Shin D., Joo K., et al. Shin H. Single-chain insulin analogs as an insulin agonist and their implications for receptor binding. Biodesign. 2015;3:168–174. [Google Scholar]
- 84.Papaioannou A., Kuyucak S., Kuncic Z. Elucidating the activation mechanism of the insulin-family proteins with molecular dynamics simulations. PLoS One. 2016;11:e0161459. doi: 10.1371/journal.pone.0161459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pandyarajan V., Phillips N.B., et al. Weiss M.A. Contribution of TyrB26 to the function and stability of insulin: structure-activity relationships at a conserved hormone-receptor interface. J. Biol. Chem. 2016;291:12978–12990. doi: 10.1074/jbc.M115.708347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi M., Sasaoka T., et al. Shigeta Y. Receptor binding and biologic activity of biosynthetic human insulin and mini-proinsulin produced by recombinant gene technology. Diabetes Res. Clin. Pract. 1989;7:25–28. doi: 10.1016/0168-8227(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 87.Papaioannou A., Kuyucak S., Kuncic Z. Computational study of the activity, dynamics, energetics and conformations of insulin analogues using molecular dynamics simulations: application to hyperinsulinemia and the critical residue B26. Biochem. Biophys. Rep. 2017;11:182–190. doi: 10.1016/j.bbrep.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Křížková K., Veverka V., et al. Žáková L. Structural and functional study of the GlnB22-insulin mutant responsible for maturity-onset diabetes of the young. PLoS One. 2014;9:e112883. doi: 10.1371/journal.pone.0112883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hua Q.X., Shoelson S.E., et al. Weiss M.A. Receptor binding redefined by a structural switch in a mutant human insulin. Nature. 1991;354:238–241. doi: 10.1038/354238a0. [DOI] [PubMed] [Google Scholar]
- 90.Rege N.K., Liu M., et al. Weiss M.A. Register-shift” insulin analogs uncover constraints of proteotoxicity in protein evolution. J. Biol. Chem. 2020;295:3080–3098. doi: 10.1074/jbc.RA119.011389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ludvigsen S., Olsen H.B., Kaarsholm N.C. A structural switch in a mutant insulin exposes key residues for receptor binding. J. Mol. Biol. 1998;279:1–7. doi: 10.1006/jmbi.1998.1801. [DOI] [PubMed] [Google Scholar]
- 92.Sims E.K., Carr A.L.J., et al. Evans-Molina C. 100 Years of insulin: celebrating the past, present and future of diabetes therapy. Nat. Med. 2021;27:1154–1164. doi: 10.1038/s41591-021-01418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flier J.S., Kahn C.R. Insulin: a pacesetter for the shape of modern biomedical science and the Nobel Prize. Mol. Metabol. 2021;52:101194. doi: 10.1016/j.molmet.2021.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brange J., Andersen L., et al. Rasmussen E. Toward understanding insulin fibrillation. J. Pharmacol. Sci. 1997;86:517–525. doi: 10.1021/js960297s. [DOI] [PubMed] [Google Scholar]
- 95.Sun H., Saeedi P., et al. Magliano D.J. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogle G.D., James S., et al. Patterson C.C. Global estimates of incidence of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res. Clin. Pract. 2022;183:109083. doi: 10.1016/j.diabres.2021.109083. [DOI] [PubMed] [Google Scholar]
- 97.Weiss M. Design of ultra-stable insulin analogues for the developing world. J. Health Spec. 2013;1:59. [Google Scholar]
- 98.Jarosinski M.A., Chen Y.S., et al. Weiss M.A. New horizons: next-generation insulin analogues: structural principles and clinical goals. J. Clin. Endocrinol. Metab. 2022;107:909–928. doi: 10.1210/clinem/dgab849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li S., Brown M.S., Goldstein J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Semple R.K., Sleigh A., et al. Savage D.B. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jarosinski M.A., Dhayalan B., et al. Weiss M.A. ‘Smart’ insulin-delivery technologies and intrinsic glucose-responsive insulin analogues. Diabetologia. 2021;64:1016–1029. doi: 10.1007/s00125-021-05422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The coordinates of the structures determined here and their associated structure factors have been deposited in the Protein Data Bank (accession code PDB: 7KD6). Molecular dynamics and modeling data supporting this work can be accessed from the La Trobe University FigShare repository: https://opal.latrobe.edu.au/projects/Single-chain_Insulin_Analogs_Threaded_by_the_Insulin_Receptor_CT_Domain/147667.