Abstract
Background
Suppression of tumorigenicity 2 (ST2) is a member of the interleukin (IL)-1 family and has 2 isoforms: ST2L, a transmembrane form, and ST2, a soluble form. IL-33 can act as an immune system alarm signal when released by damaged cells, which in turn activates other cells expressing the ST2 receptor. This can cause inflammatory cytokines to be released and produced, as well as trigger osteoclastogenesis. This study aimed to investigate the levels of soluble ST2 in gingival samples.
Material/Methods
The study population consisted of 30 individuals. The participants were divided into 3 groups: healthy participants, patients with periodontitis, and patients with periodontitis and diabetes mellitus. Periodontitis was determined using probing depth, clinical attachment loss, and gingival index. Patients with stage 2 to 4 periodontitis met the inclusion criteria. Gingival crevicular fluid (GCF) was collected for quantification of samples for ST2 levels by using an enzyme immunoassay.
Results
The mean±standard deviation of ST2 GCF concentrations was relatively high (558.87±68.99) in the group with periodontitis and diabetes mellitus, compared with that of the periodontitis group (452.06±54.18) and healthy group (252.82±87.9).
Conclusions
GCF ST2 values were found to be a marker of inflammatory activities. Thus, GCF ST2 could be a potential biomarker for the diagnosis of periodontitis as well as systemic diseases, such as diabetes mellitus. This pilot study was limited by a small number of participants. To confirm the associations, more large-scale investigations should be conducted.
Keywords: Chronic Periodontitis, Diabetes Mellitus, Gingival Crevicular Fluid
Background
Periodontitis is a multifactorial disease defined by microbially induced, host-mediated inflammation that leads to periodontal attachment loss and tooth loss [1]. An increased risk for periodontitis has been shown with the variations in genes related to the inflammatory response [2]. Diabetes mellitus is a progressive metabolic disease and a great threat to human health. According to the International Diabetes Federation, 463 million people worldwide were diagnosed with diabetes in 2019. By 2030, 578 million people are expected to have diabetes, with this figure expected to rise 51% by 2045 [3]. The major complications of diabetes are diabetic nephropathy, diabetic neuropathy, cardiomyopathy, delayed wound healing, and retinopathy. The sixth complication of diabetes mellitus is periodontitis [4]. The link between periodontitis and diabetes mellitus is a “double-edged sword”, with uncontrolled diabetes leading to periodontal destruction and loss of teeth. On the other hand, poor periodontal health leads to insulin resistance and diabetes mellitus [5].
The pathogenic mechanism between periodontitis and diabetes is the chronic elevation of proinflammatory mediators, such as interleukin (IL) 1 beta [6] and tumor necrosis factor (TNF) alpha in serum, leading to insulin resistance. Chronic elevations in blood glucose levels in patients with diabetes and the accumulation of advanced glycation end products in periodontal tissues lead to periodontal destruction and worsening of the periodontal condition [5]. The levels of soluble Suppression of tumorigenicity 2 (ST2) and C-reactive protein in the heart rise in response to inflammation and infection. ST2 belongs to the interleukin-1 family and has 2 isoforms: ST2L, which is a transmembrane form, and ST2S, which is a soluble form [7]. When injured cells release IL-33, it acts as an immune system alarm signal, activating other cells that carry the ST2 receptor [8]. This can cause inflammatory cytokines to be released and produced, as well as trigger osteoclastogenesis [9]. Increased levels of serum ST2 have been linked to a poor outcome in patients with coronary heart disease during clinical investigations of heart failure [10,11]. ST2L has been found to reduce macrophage foam cell formation when it interacts with IL-33 [12], whereas serum ST2 counteracts the functions of ST2L. Although the fact that periodontitis is linked to changes in inflammatory markers suggests that periodontal changes have a systemic influence, one research study has looked at the link between periodontitis and changed levels of ST2. The scientific evidence for this link is currently equivocal, and reductions in ST2 levels are rarely observed following periodontal therapy. Furthermore, practically all research has looked at the link between cardiovascular disease, diabetes, inflammatory bowel diseases, sepsis, and ST2, but only one study has looked at ST2 levels in periodontitis patients [13–18]. There has been no research on ST2 levels concerning metabolic and inflammatory illnesses collected from the gingival crevicular fluid (GCF) [19]. Based on these reasons, ST2 is currently being studied as a new marker of systemic inflammation. Therefore, the goal of this study was to investigate the expression of ST2 in patients with periodontitis and diabetes mellitus, without any systemic complications.
Material and Methods
The study was approved by the Institutional Review Board of Saveetha Dental College and Hospital, Chennai, India (IEC approval no. IHEC/SDC/PERIO-2005/22/421).
Thirty participants (17 men and 13 women) was stratified into 3 groups: group 1 (n=10), healthy participants; group 2 (n=10), patients with periodontitis; and group 3 (n=10), patients with periodontitis and diabetes mellitus.
The inclusion criteria were as follows: Patients with stage 2 to 4 periodontitis (according to the World Workshop of Periodontics classification, 2017) [20]; probing depth of 6 mm or more; clinical attachment loss of 3 mm to 5 mm; radiographic bone loss of the coronal third and extending to mid-third of root and beyond; tooth loss due to periodontitis of 5 or more teeth, and vertical bone loss of more than 3 mm; and type 2 diabetes mellitus (HbA1c values ranging from 6.8 to 7.5 were included).
The exclusion criteria were as follows: smoking; type 1 diabetes mellitus; other systemic diseases apart from diabetes mellitus, such as cardiovascular disease; pregnancy or lactation; patients who underwent periodontal surgery within the past 6 months; and patients on anti-inflammatory drug therapy.
Procedure (Procurement of GCF)
All participants gave their informed consent. After recording the complete periodontal chart, gentle supragingival scaling was done in the first appointment. After proper isolation, GCF was collected from deep-pocket regions in periodontitis patients and at the sulcular entrance of the tooth for healthy participants by inserting Hirschfield microcapillary pipettes (Sigma Aldrich). The amount of GCF collected was around 0.5 to 1.0 μL [21].
Transportation and Storage of GCF
The collected GCF from multiple sites of the same patient was pooled together and transferred to sterile Eppendorf tubes by convenient air blast using a chip blower and immediately stored at −80 until further investigation. The storage and assay procedures were carried out in the Blue Lab, Department of Dental Research, Saveetha Dental College. Enzyme-linked immunosorbent assay (ELISA) was performed after the completion of sample collection.
Assay Procedure
Assessment of Human ST2 Protein Levels by Quantitative Sandwich ELISA Method
In this study, ST2 protein levels were measured by a human GlycA quantitative sandwich ELISA kit (MyBioSource Inc USA; San Diego, CA, USA), as per the manufacturer protocol. Briefly, standard and testing sample wells were set and 50 μL of serially diluted standards were added to standard wells. In the test sample wells, 40 μL of sample diluents were added into sample wells, then 10 μL of the test sample was added. Next, the plates were covered and incubated for 45 min at 37°C. After the incubation, the wells were washed with a washing buffer (250 μL) 5 times each at intervals of 1 to 3 min. After the last wash, the remaining washing buffer was removed by aspirating or decanting, and then the plate was inverted and blotted against clean paper towels. Then, 50 μL of horseradish peroxide-conjugated detection antibody was added to each well (except blank well), covered with a plate cover, and incubated for 30 min at 37°C. After incubation, the washing steps were repeated to remove the excess antibody. An amount of 50 μL chromogen solution A and 50 μL chromogen solution B were added to each well and gently mixed and incubated for 15 min at 37°C in the dark, to protect from light. After 50 μL of stop solution was added to each well, the optical density was read at 450 nm using a microtiter plate reader within 15 min. The concentration of ST2 was expressed as pg/L against human GCF standards. The detection range of the kit is 156 to 10 000 pg/L, and the sensitivity of the assay is from 94 pg to 10 000 pg/L [22].
Statistical Analyses
All data collected in the present study was expressed in the form of mean±standard deviation, and statistical significance was assessed using SPSS version 23.0 for Windows (IBM Corp, Armonk, NY, USA). The chi-square test was used to assess statistically significant differences between groups in age, sex, and periodontal status, with and without diabetes mellitus. One-way ANOVA was performed to determine statistical significance between groups. Linear regression analysis with odds ratios was performed.
Results
According to the findings of this investigation, group 3 had the highest mean glycA content in GCF (Figure 1). Table 1 shows the descriptive statistics of the study groups. One-way ANOVA was used to analyze the statistical difference between groups, with a P value less than 0.05 indicating a statistically significant difference in expression between the groups, as shown in Table 1. The mean ST2 concentrations obtained are shown in Table 2. The study population consisted of 30 participants with an age range of 20 to 60 years, out of which 17 were men and 13 were women. There was no difference between participant sex and the expression of ST2, as shown in Table 3. The mean differences between different groups are shown in Figure 1. Multiple comparisons of the groups which were significant were further confirmed by doing a post hoc test, which showed the difference between each group was significant (Table 4). The highest mean difference was in group 3 (periodontitis+diabetes mellitus), the second-highest was in group 2 (periodontitis), and the lowest mean difference was seen in group 1 (healthy), as seen in Table 4. The linear regression equation for ST2 is {y=0.33+0.01*x}, where y=periodontal probing depth and x=ST2, with a fitness of model R2=0.864. There was a 13.354 odds risk for increased severity of periodontitis with increased ST2 (Table 5). A scatter plot with the equation is shown in Figure 2.
Figure 1.
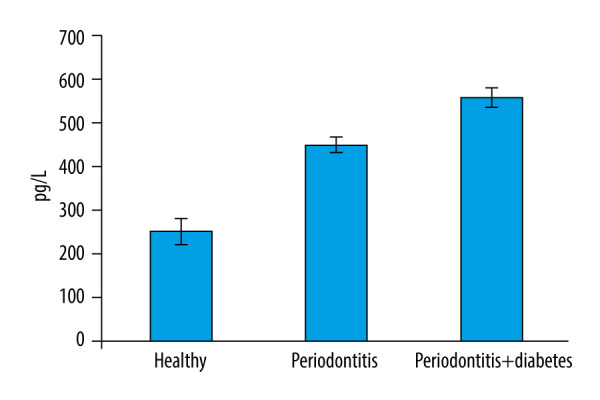
Bar graph depicts the mean suppression of tumorigenicity 2 (ST2) expression in the healthy, periodontitis, and periodontitis with diabetes mellitus groups.
Table 1.
Descriptive statistics of baseline parameters in the study population.
| Group | Sex (male: female) | Age (years; mean±SD) | Gingival index (mean±SD) | Probing depth (mm; mean±SD) | Clinical attachment loss (mm; mean±SD) | Gingival crevicular fluid (pg/mL; mean±SD) |
|---|---|---|---|---|---|---|
| Group 1 | 5: 5 | 23.60±0.788 | 0 | 2.53±0.808 | 0 | 66.34±0.109 |
| Group 2 | 5: 3 | 32.12±5.12 | 2.09±0.55 | 2.54±0.98 | 7±2.234 | 64.22±0.89 |
| Group 3 | 7: 5 | 35.66±8.69 | 2.76±0.344 | 6.54±1.89 | 8±2.267 | 103.66±1.08 |
| P value | Not applicable | Not applicable | <0.0012 | <0.001 | <0.0018 | 0.001±0.02 |
P<0.05, statistically significant difference between groups.
Table 2.
Comparative analysis of levels of suppression of tumorigenicity 2 (ST2) between groups using one-way ANOVA.
| Serial no. | Data | Mean±standard deviation (pg/L) | P value |
|---|---|---|---|
| 1 | Group 1 | 252.82±87.91 | 0.000* |
| Group 2 | 452.06±54.18 | ||
| 2 | Group 2 | 452.06±54.18 | 0.000* |
| Group 3 | 558.87±68.99 | ||
| 3 | Group 3 | 558.87±68.99 | 0.000* |
| Group 1 | 252.82±87.91 |
Group 1, healthy; group 2, periodontitis; group 3, periodontitis with diabetes mellitus.
P<0.05, statistically significant difference between groups.
Table 3.
Comparison of suppression of tumorigenicity 2 (ST2) levels in gingival crevicular fluid (GCF) by sex and group.
| Serial no. | Sex | Group | Mean | P value |
|---|---|---|---|---|
| 1 | Male | 1 | 211.02 | 0.098 (NS) |
| 2 | 471.10 | |||
| 3 | 566.93 | |||
| 2 | Female | 1 | 315.52 | 0.078 (NS) |
| 2 | 423.38 | |||
| 3 | 550.80 |
Group 1, healthy; group 2, periodontitis; group 3, periodontitis with diabetes mellitus. NS – not significant. When the P value is more than 0.05, there is no statistical difference between the sex and ST2 expression.
Table 4.
Multiple comparisons using post hoc tests.
| Dependent variable ST2 | Group (I) | Group (J) | Mean difference (I-J) | Std error | Sig. | 95% Confidence Lower bound | Interval Upper bound |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Tukey test | Healthy | P | −200.916* | 31.19615 | .000 | −278.2647 | −123.5681 |
| P+DM | −303.559* | 32.09114 | .000 | −383.1271 | −223.9925 | ||
|
| |||||||
| Periodontitis | healthy | 200.916* | 31.19615 | .000 | 123.5681 | 278.2647 | |
| P+DM | −102.643* | 32.80524 | .011 | −183.9813 | −21.3055 | ||
|
| |||||||
| Periodontitis+ DM | Healthy | 303.559* | 32.09114 | .000 | 223.9925 | 383.1271 | |
| periodontitis | 102.643* | 32.80524 | .011 | 21.3055 | 183.9813 | ||
|
| |||||||
| Dunnett 2-sided test | Periodontitis | Healthy | 200.916* | 31.19615 | .000 | 127.9740 | 273.8589 |
|
| |||||||
| Periodontitis+ DM | Healthy | 303.559* | 32.09114 | .000 | 228.5247 | 378.5949 | |
The mean difference is significant at the 0.05 level.
Dunnett-test treats 1 group as a control and compares all other groups against it.
DM – diabetes mellitus.
Table 5.
Linear regression analysis to predict the severity of periodontitis using suppression of tumorigenicity 2 (ST2) levels in the gingival crevicular fluid (GCF).
| Independent variable | Dependent variable | Correlation coefficient (r) | β-coefficient | P value | 95% confidence interval |
|---|---|---|---|---|---|
| ST2 | Periodontal probing depth | 0.930 | 0.014 | 0.000 | −1.317 to 0.653 |
Figure 2.
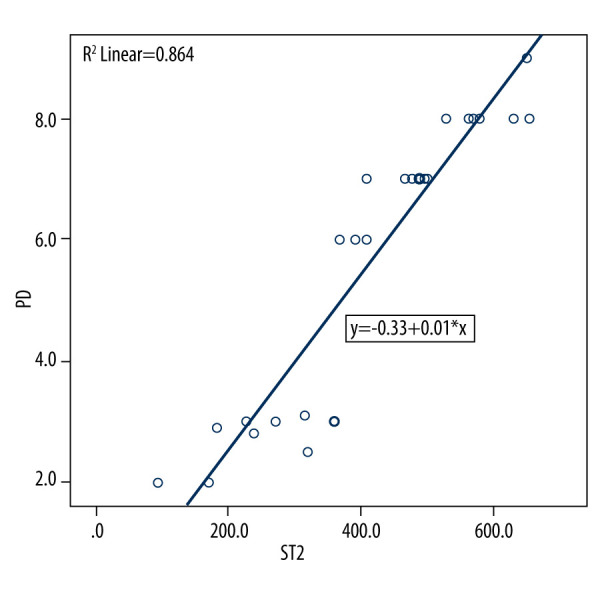
Scatter plot depicting linear regression equation to predict periodontal probing depth using suppression of tumorigenicity 2 (ST2) in gingival crevicular fluid (GCF). y – periodontal probing depth; x – ST2; PD – probing depth.
Discussion
Periodontitis, as we know, is a chronic infectious inflammatory disease, in which the interaction between host and pathogen activates proinflammatory cascade reactions. This results in the secretion of TNF alpha and interleukin 1 beta, which in turn causes connective tissue destruction and bone resorption. These increased pro-inflammatory cytokines induce insulin resistance and decrease insulin action, which in turn causes the accumulation of advanced glycation end-products (AGE). AGE proteins react with macrophage AGE receptors, and this interaction causes the synthesis and secretion of TNF alpha and interleukin 1 beta, which leads to increased oxidative stress. Thus, increased cascade reactions cause connective tissue degradation and activation of collagenases and MMPs. In the same way, increased insulin resistance leads to degradation of the connective tissue and activation of MMPs, and accumulation of additional AGE proteins, which results in thrombosis and release of more proinflammatory cytokines. Because of accumulation and thrombosis, there is a decreased migration of neutrophils and polymorphonuclear neutrophils, thus causing the microflora to invade more and cause more tissue destruction [23]. Thus periodontitis and diabetes have a 2-way relationship.
Many studies have been done to determine the role of ST2 in inflammatory diseases.
Boga et al conducted a study to find the correlation between serum ST2 and Crohn disease by using clinical, endoscopic, and histopathological activity. They found that serum ST2 levels showed increased levels from healthy to inflammatory disease conditions [24]. Similarly, another study showed that diabetes mellitus, atrial fibrillation, renal dysfunction, right ventricular pressure overload, dysfunction, systemic congestion, and exercise intolerance were all linked to increased serum ST2 levels [25]. Another study found similar results, showing that serum ST2 and IL-17 were upregulated in rheumatoid arthritis, whereas IL-10 was downregulated [26]. The present study showed similar results of increased serum ST2 levels in chronic inflammatory conditions, namely periodontitis.
Studies have proven that type 2 diabetes mellitus is associated with increased levels of serum ST2, with a 2- to 4-fold increase in the risk of diabetes [27]. A case-control study concluded that serum ST2 is not only associated with diabetes but also with increased thickness of carotid intima-media in type 2 diabetes mellitus. Serum ST2 might act as a potential marker for the macrovascular complications of diabetes mellitus [27,28]. The present study results supported the results of the previously mentioned study, because there was an increase in serum ST2 levels in the diabetes mellitus and periodontitis group.
The literature shows only one study was done on serum ST2 levels linked to periodontitis which shows both serum ST2 and C- reactive protein levels were increased in patients with periodontitis, compared with the healthy population [13]. However, the study was done only on periodontitis and control groups and did not mention diabetes mellitus and its correlation with serum ST2. The present study aimed to estimate the serum ST2 levels in patients with periodontitis and patients with periodontitis with diabetes mellitus. Our results showed that the mean value of ST2 in GCF was significantly high in group 3 (periodontitis+diabetes mellitus). There was a significant difference seen between groups 1 (healthy) and 2 (periodontitis without diabetes mellitus), as shown in Figure 1, whereby group 2 showed higher serum ST2 levels than group 1. This shows that periodontitis might not be directly related to the elevated ST2, but instead plays an indirect role in elevating ST2 biomarkers by increasing inflammatory infiltrates, as explained by a 2-way relationship. In the present study, periodontitis along with diabetes mellitus caused more inflammation and destruction than periodontitis alone. These results agree with the previous study results [13].
This study had limitations, including that it was a pilot study with a small sample size and there was no control group for diabetes mellitus without periodontitis. Based on our study results, we plan further multicenter research to overcome these limitations, with a larger sample size and with a fourth group of patients with diabetes alone.
Conclusions
A single serum ST2 measurement may be sufficient for titrating therapy and assessing a patient’s clinical condition. However, ST2 should be studied for further consideration as a diagnostic target. Thus, ST2 in the GCF may act as a potential marker of inflammatory activity in diabetes mellitus. This is the only study that we are aware of that estimated and compared ST2 levels in the GCF with periodontitis and diabetes mellitus. In this study, the greater amount of periodontal tissue destruction along with a metabolic disorder resulted in a higher mean ST2 concentration. After further investigation, we believe ST2 could be used as a biomarker. Future research with a large sample size needs to be conducted.
Footnotes
Conflict of interest: None declared
Ethics Approval
The study was conducted following the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Saveetha Dental College and Hospital, Chennai, India (protocol code IHEC/SDC/PERIO-2005/22/421).
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Newman MG, Carranza FA, Klokkevold PR, Takei HH. Newman and Carranza’s clinical periodontology e-book. Elsevier Heal Sci. 2018 [Google Scholar]
- 2.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–87. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 5.Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mootha A, Malaiappan S, Milstein DMJ, et al. Comparison of interleukin-21 levels and its correlation with clinical parameters among healthy individuals, chronic periodontitis, and aggressive periodontitis patients. J Clin Transl Res. 2021;7:84–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Ciccone M, Cortese F, Gesualdo M, et al. A novel cardiac biomarker: ST2: A review. Molecules. 2013;18:15314–28. doi: 10.3390/molecules181215314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107(17):8017–22. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bignardi LA. Estudo do papel do eixo IL-33/ST2 na progress ão da lesão periapical experimental. Universidade de São Paulo; 2014. [in Portuguese] [DOI] [Google Scholar]
- 10.Brint EK, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–79. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 11.Dieplinger B, Egger M, Haltmayer M, et al. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: Results from the ludwigshafen risk and cardiovascular health study. Clin Chem. 2014;60:530–40. doi: 10.1373/clinchem.2013.209858. [DOI] [PubMed] [Google Scholar]
- 12.McLaren JE, Michael DR, Salter RC, et al. IL-33 reduces macrophage foam cell formation. J Immunol. 2010;185:1222–19. doi: 10.4049/jimmunol.1000520. [DOI] [PubMed] [Google Scholar]
- 13.Torrungruang K, Katudat D, Mahanonda R, et al. Periodontitis is associated with elevated serum levels of cardiac biomarkers – soluble ST2 and C-reactive protein. J Clin Periodontol. 2019;46:809–18. doi: 10.1111/jcpe.13149. [DOI] [PubMed] [Google Scholar]
- 14.Vachhani KS, Bhavsar NV. Effects of non-surgical periodontal therapy on serum inflammatory factor high-sensitive C-reactive protein, periodontal parameters and renal biomarkers in patients with chronic periodontitis and chronic kidney disease. Dent Med Probl. 2021;58(4):489–98. doi: 10.17219/dmp/136034. [DOI] [PubMed] [Google Scholar]
- 15.Khudan R, Krynytska I, Marushchak M, Korda M. Influence of chronic hyperhomocysteinemia on the features of bone metabolism in the case of lipopolysaccharide-induced periodontitis. Dent Med Probl. 2022;59(2):255–61. doi: 10.17219/dmp/143948. [DOI] [PubMed] [Google Scholar]
- 16.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–94. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 17.Burakoff R, Pabby V, Onyewadume L, et al. Blood-based biomarkers used to predict disease activity in Crohn’s disease and ulcerative colitis. Inflammatory bowel diseases. 2015;21(5):1132–40. doi: 10.1097/MIB.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 18.Brunner M, Krenn C, Roth G, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004;30(7):1468–73. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 19.Yiğit U, Kırzıoğlu FY, Özmen Ö, Uğuz AC. Protective effects of caffeic acid phenethyl ester on the heart in experimental periodontitis against oxidative stress in rats. Dent Med Probl. 2021;58(3):335–41. doi: 10.17219/dmp/132388. [DOI] [PubMed] [Google Scholar]
- 20.Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89:S173–82. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 21.Karthikeyan BV, Pradeep AR. Leptin levels in gingival crevicular fluid in periodontal health and disease. J Periodont Res. 2007;42(4):300–4. doi: 10.1111/j.1600-0765.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- 22.Bignardi LA. Doctoral dissertation. Universidade de São Paulo; Estudo do papel do eixo IL-33/ST2 na progress ão da lesão periapical experimental. [in Portuguese] [Google Scholar]
- 23.Kaarthikeyan G, Jayakumar ND, Padmalatha O, et al. Analysis of the association between interleukin-1β (3954) gene polymorphism and chronic periodontitis in a sample of the south Indian population. Indian J Dent Res. 2009;20:37. doi: 10.4103/0970-9290.49061. [DOI] [PubMed] [Google Scholar]
- 24.Boga S, Alkim H, Koksal AR, et al. Serum ST2 in inflammatory bowel disease: A potential biomarker for disease activity. J Investig Med. 2016;64:1016–24. doi: 10.1136/jim-2016-000062. [DOI] [PubMed] [Google Scholar]
- 25.AbouEzzeddine OF, McKie PM, Dunlay SM, et al. Soluble ST2 in heart failure with preserved ejection fraction. J Am Heart Assoc. 2017;6:e004382. doi: 10.1161/JAHA.116.004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Chen Z, Huang Y, et al. Prognostic significance of serum interleukins and soluble ST2 in traditional Chinese medicine (TCM) syndrome-differentiated rheumatoid arthritis. Med Sci Monit. 2018;24:3472–78. doi: 10.12659/MSM.907540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y-H, Zhang R-C, Hou L-B, et al. Distribution and clinical association of plasma soluble ST2 during the development of type 2 diabetes. Diabetes Res Clin Pract. 2016;118:140–45. doi: 10.1016/j.diabres.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Zhang H, Song Y, et al. Soluble ST2 is associated with increased carotid intima-media thickness in patients with type 2 diabetes mellitus. Medicine (Baltimore) 2020;99:e18940. doi: 10.1097/MD.0000000000018940. [DOI] [PMC free article] [PubMed] [Google Scholar]


