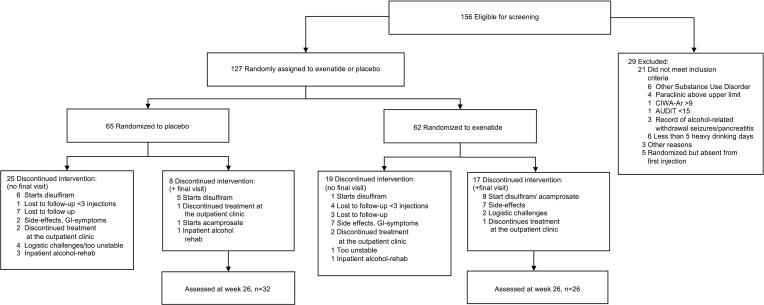
Figure 1. CONSORT flow diagram.
Study diagram of patient flow according to CONSORT 2010 statement. Details regarding initial meetings and ineligibility for screening can be found in Supplemental Figure 3, and a flowchart for the 6-month follow-up can be found in Supplemental Figure 1. Of the 127 patients included in the study, 65 patients were randomized to 2 mg exenatide once weekly, and 62 patients were randomized to placebo. Thirty-two patients from the exenatide group and 26 patients from the placebo group completed the study after 26 weeks of trial participation. AUDIT, Alcohol Use Disorders Identification Test; CIWA-Ar, Clinical Institute Withdrawal Assessment for Alcohol, Revised.