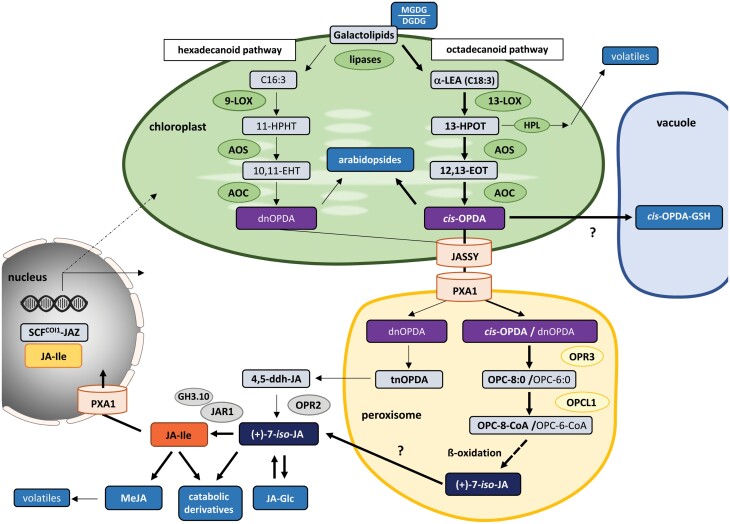
Fig. 4.
The role of organelles in jasmonate signaling. The term jasmonate comprises JA and JA-Ile as well as several precursors and catabolic derivatives some of which also possess bioactivity. Jasmonate biosynthesis is initiated in chloroplasts by oxidation of C18:3 (octadecanoid pathway) and C16:3 (hexadecanoid pathway) fatty acids derived from galactolipids, which are converted in several steps to the first committed precursor, OPDA. The MDGD/DGDG ratio, but also conjugation of OPDA to GSH or esterification to galactolipids (arabidopsides), affects OPDA homeostasis. Biosynthesis of JA continues in peroxisomes by β-oxidation of OPC-8:0 and OPC-6:0. A minor, less well described bypass pathway of JA formation involves tnOPDA and 4,5-ddh-JA. JA-Ile, the most bioactive of the jasmonates, is finally synthesized in the cytosol from JA and isoleucine, the latter being derived from methionine also made in chloroplasts. Ultimately, JA-Ile exerts its action in the nucleus by promoting the formation of SCFCOI1–JAZ co-receptor complexes and thus releasing JAZ-dependent gene suppression. 4,5ddh-JA, 4,5-didehydro-jasmonate; 10,11-EHT, 10,11(S)-epoxy-hexadecatrienoic acid; 11-HPHT, 11(S)-hydroperoxy-hexadecatrienoic acid; 12,13-EOT, 12,13(S)-epoxy-octadecatrienoic acid; 13-HPOT, 13(S)-hydroperoxylinolenic acid; α-LEA, α-linolenic acid; AOC, allene oxide cyclase; AOS, allene oxide synthase; DGDG, digalactosyl-diacylglycerol; GH3.10, glycoside hydrolase 3 gene family 10; HPL, hydroperoxide lyase; JA, jasmonic acid; JA-Glc, glycosylated jasmonate; JAR1, jasmonate-resistant 1; JASSY, chloroplast jasmonate transporter; LOX, lipoxygenases; MeJA, methyl jasmonate; MGDG, monogalactosyldiacylglycerol; OPC, 3-oxo-2-(20-[Z]-pentenyl)-cyclopentane-1-octanoic acid; OPCL1, OPC-8:0 CoA ligase 1; OPDA, oxophytodienoic acid; OPR, OPDA reductase; PXA1, peroxisomal ABC-transporter 1.