Figure 2. SPP cleaves mislocalized mitochondrial TA proteins during ERAD.
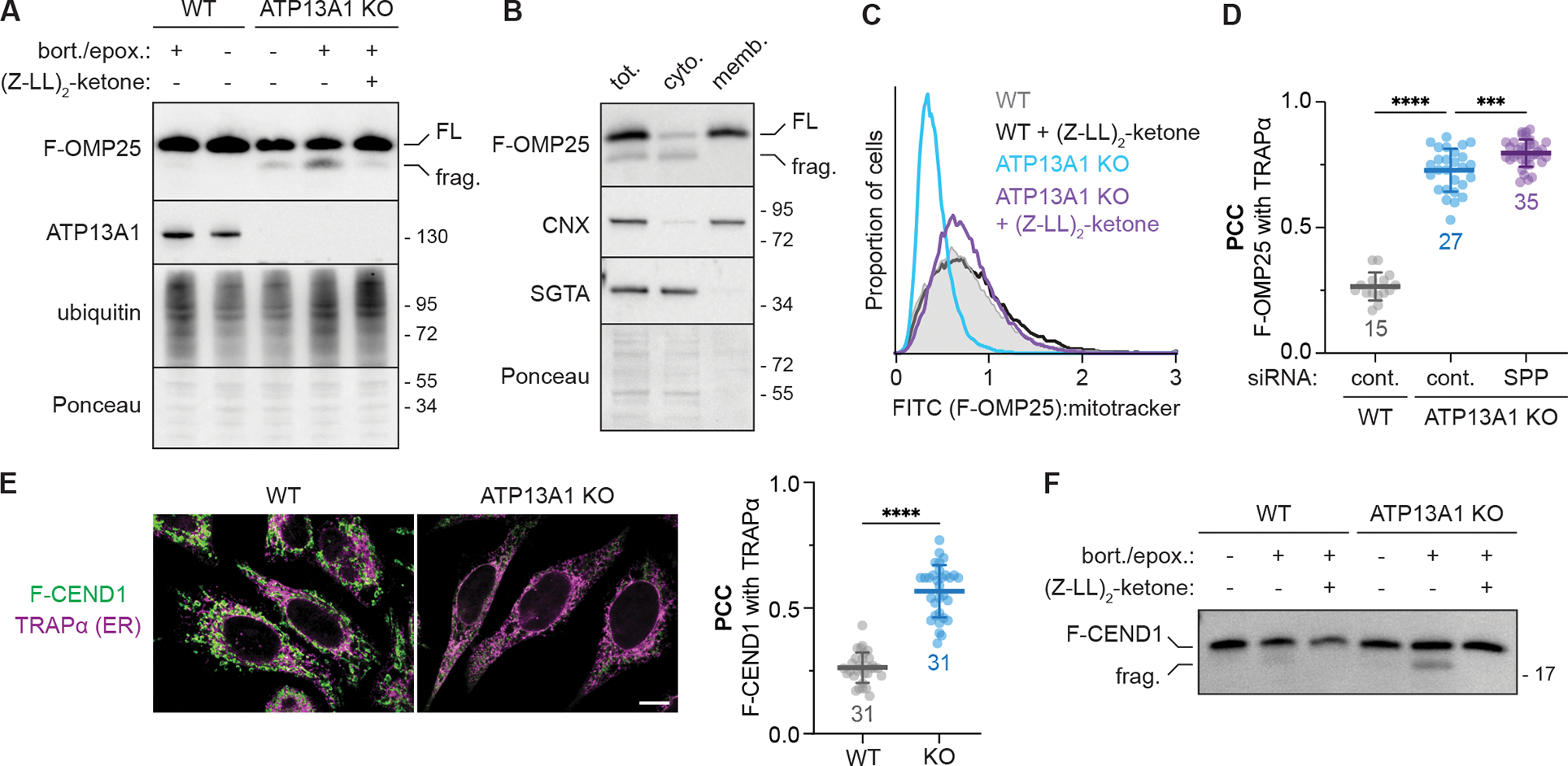
(A) Proteasome inhibition stabilizes an SPP-dependent fragment of mislocalized F-OMP25. SDS-PAGE and immunoblotting for the indicated factors in wildtype (WT) and ATP13A1 knockout (KO) cells treated without or with proteasome inhibitors (0.5 μM bortezomib and 0.5 μM epoxomicin; bort./epox.) and/or an inhibitor [5 μM (Z-LL)2-ketone] of the signal peptide peptidase (SPP). FL, full-length F-OMP25; frag., F-OMP25 fragment.
(B) SPP-cleaved F-OMP25 is cytosolic. Immunoblotting of lysates (tot.) of ATP13A1 KO cells expressing F-OMP25 treated with bort./epox. and separated into cytosolic (cyto.) and membrane-bound (memb.) fractions.
(C) SPP inhibition stabilizes mislocalized F-OMP25. Fluorescent flow cytometry of F-OMP25 levels normalized to mitotracker staining in WT or ATP13A1 KO cells treated without or with 5 μM (Z-LL)2-ketone.
(D) SPP depletion does not rescue mitochondrial TA protein localization. Pearson’s correlation coefficients (PCC; mean ± sd and individual points for indicated sample size) measuring the colocalization of F-OMP25 and the ER marker TRAPα in WT or ATP13A1 KO cells treated with control (cont.) or SPP siRNAs as in Figure S2G. ****, p<0.0001; ***, p<0.0003. Cont. siRNA samples are a subset of Figure 1B.
(E) CEND1 is an ATP13A1-dependent mitochondrial TA protein. Immunofluorescence (left) and PCC (right; mean ± sd and individual points for indicated sample size) showing colocalization of a FLAG-tagged reporter containing the CEND1 TM (F-CEND1, green) and the ER marker TRAPα (magenta) in WT or ATP13A1 KO Flp-In HeLa T-REx cells. Scale bar, 10 μm; ****, p<0.0001.
(F) Mislocalized CEND1 is cleaved by SPP. SDS-PAGE and immunoblotting of soluble fractions from semi-permeabilized WT or ATP13A1 KO cells expressing F-CEND1 treated without or with proteasome inhibitors (bort./epox.) and/or (Z-LL)2-ketone.
See also Figures S2, S3, and Supplemental Table 1.
