Table 2.
Scope of Metallaphotoredox C(sp3)–C(sp3) Cross-Coupling of Carboxylic Acids and Alcoholsa
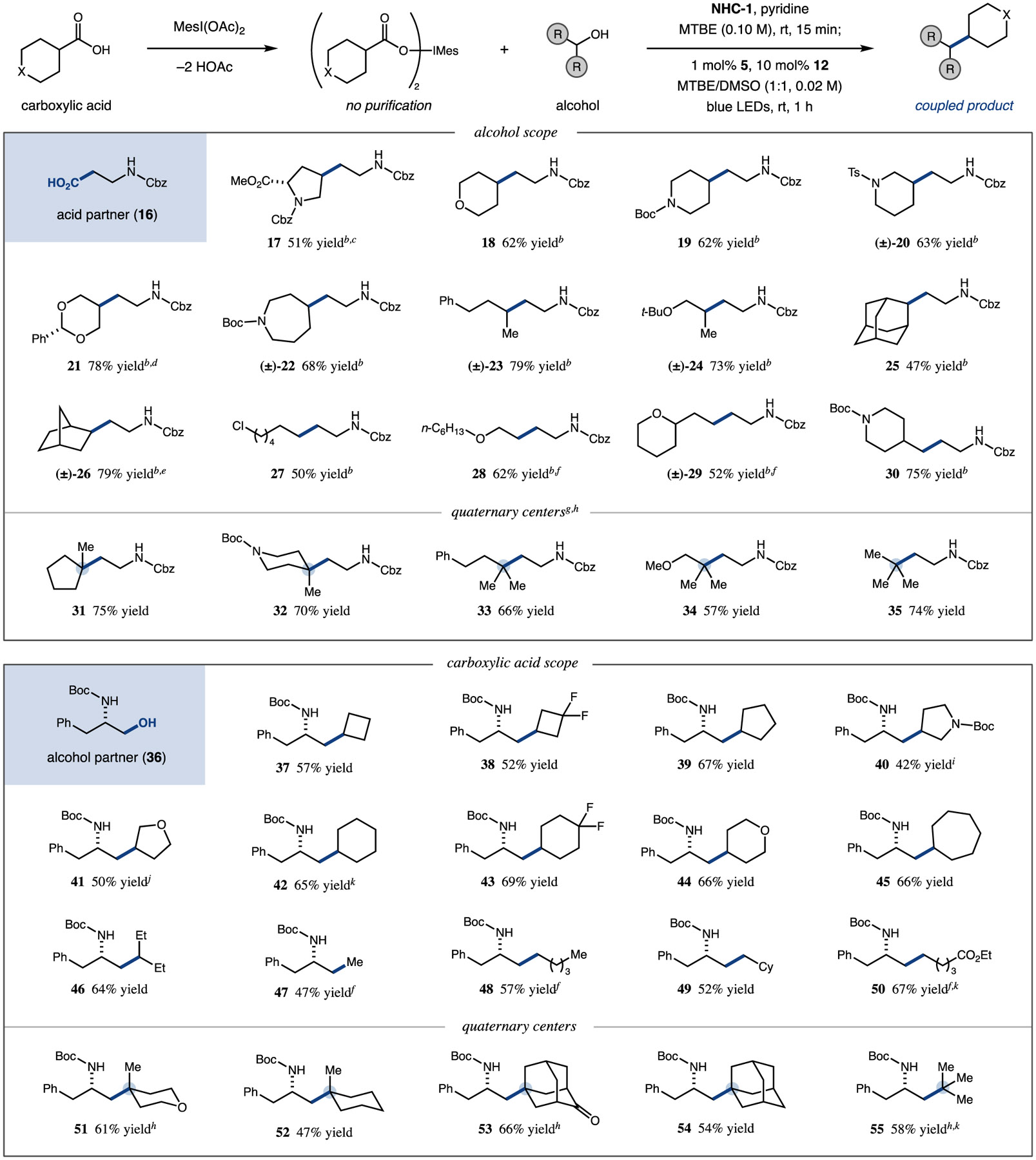
|
Iodonium dicarboxylate formed with MesI(OAc)2 (2 equiv) and carboxylic acid (4 equiv) in toluene (0.05 M) at 55 °C over 10 min. Coupling performed with alcohol substrate (0.50 mmol, 1.0 equiv), NHC-1 (1.10 equiv), and pyridine (1.05 equiv) in MTBE (0.10 M) for 15 min at room temperature, then iridium photocatalyst 5 (1.0 mol %), nickel catalyst 12 (10 mol %), and preformed iodomesitylene dicarboxylate (2.0 equiv, added over 5 min) in MTBE/DMSO (1:1, 0.02 M) with blue LED irradiation for 1 h at 23 °C. Homodimerization of the limiting alcohol substrate is typically 5–10%. Yields are isolated unless otherwise noted. See SI for experimental details.
1.30 equiv of NHC-1, 1.25 equiv of pyridine.
2.7:1 dr.
8:1 dr.
>20:1 dr.
Yield by 1H NMR.
NHC-2 in PhCF3, −20 to 0 °C for 4 h; 2 mol % 5, 20 mol % 12.
1.5 equiv iodomesitylene dicarboxylate.
1.1:1 dr.
1:1 dr.
>99% ee by HPLC.
