Figure 3.
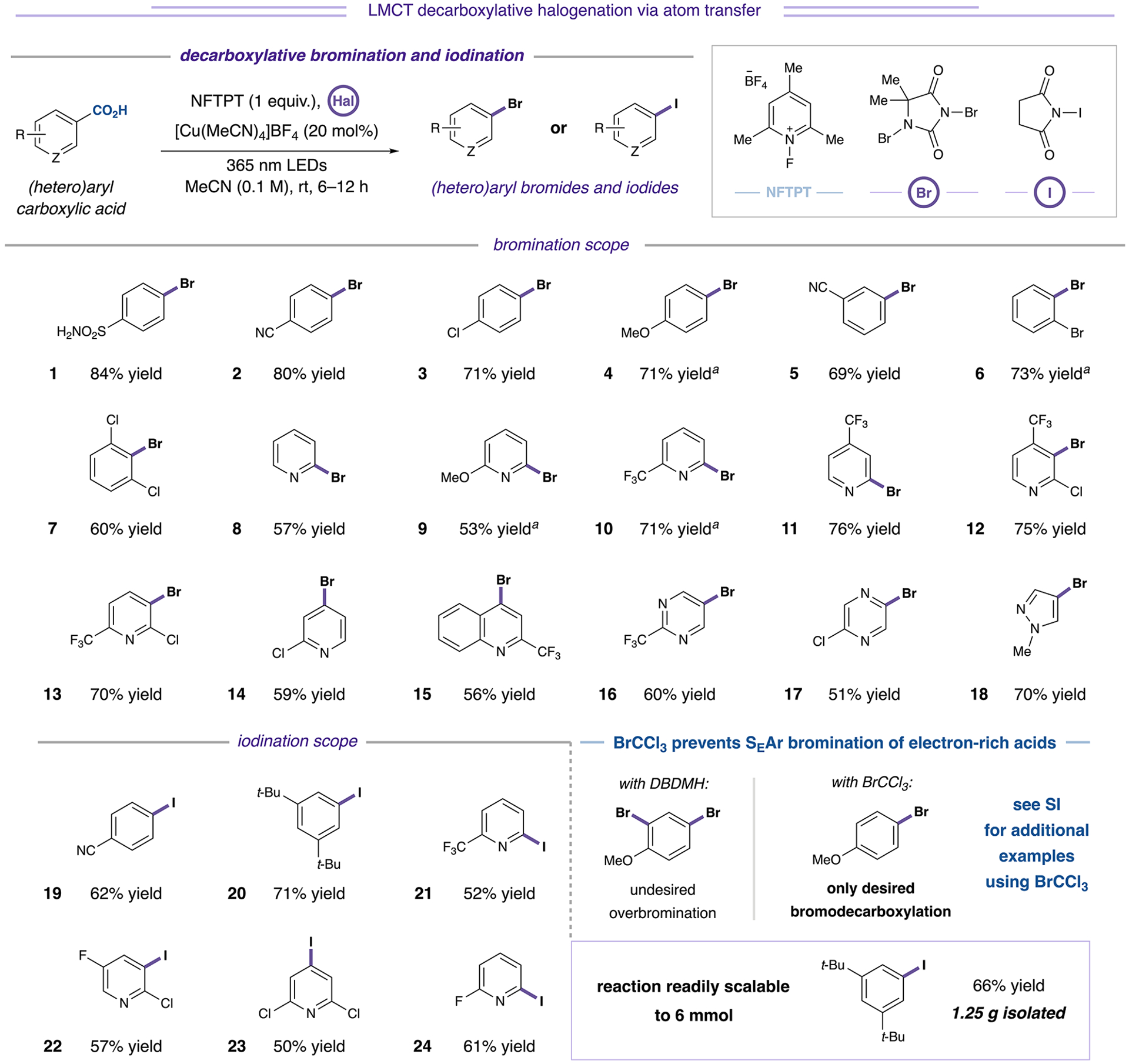
Decarboxylative bromination and iodination of (hetero)aryl carboxylic acids. Bromination conducted with 1,3-dibromo-5,5-dimethylhydantoin (0.75 equiv) unless otherwise specified. Iodination conducted with N-iodosuccinimide (1 equiv). aWith BrCCl3 (3 equiv) as bromination reagent. See Supporting Information for additional examples and experimental details.
