Table 1.
Control Experiments for LMCT Decarboxylative Bromination
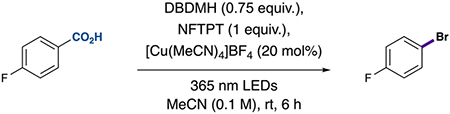
| ||
|---|---|---|
| entrya | deviations | yieldb |
| 1 | none | 72% |
| 2 | no light | 0% |
| 3 | no Cu | 0% |
| 4 | no oxidant | 0% |
| 5 | no bromination reagent | 0%c |
| 6 | with NBS (1 equiv) instead of DBDMH | 58% |
| 7 | with DCP (1 equiv) instead of NFTPT | 57% |
| 8 | with 1 equiv Cu(II) + no oxidantd | 73% |
0.1 mmol scale.
Yields determined by 1H NMR analysis.
24% yield fluorobenzene as determined by 1H NMR analysis.
Performed with 1 equiv Cu(OTf)2 and 1 equiv of 2,2,6,6-tetramethylpiperidine as base.
NBS, N-bromosuccinimide. DCP, dicumylperoxide. See Supporting Information for further details.
