Summary
Background
Continuous glucose monitoring (CGM) improves glycaemia for people affected by type 1 diabetes (T1D), but is not funded in Aotearoa/New Zealand. This study explores the impact of non-funded CGM on equity of access and associated glycaemic outcomes.
Methods
Cross-sectional population-based study collected socio-demographic (age, gender, prioritised ethnicity, socioeconomic status) and clinical data from all regional diabetes centres in New Zealand with children <15 years with T1D as of 1st October 2021. De-identified data were obtained from existing databases or chart review. Outcomes compared socio-demographic characteristics between those using all forms of CGM and self-monitoring of blood glucose (SMBG), and association with HbA1c.
Findings
1209 eligible children were evaluated: 70.2% European, 18.1% Māori, 7.1% Pacific, 4.6% Asian, with even distribution across socioeconomic quintiles. Median HbA1c was 64 mmol/mol (8.0%), 40.2% utilised intermittently scanned (is)CGM, and 27.2% real-time (rt)CGM. CGM utilisation was lowest with Pacific ethnicity (38% lower than Māori) and the most deprived socioeconomic quintiles (quintile 5 vs. 1 adjusted RR 0.69; 95% CI, 0.57 to 0.84). CGM use was associated with regional diabetes centre (P < 0.001). The impact of CGM use on HbA1c differed by ethnicity: Māori children had the greatest difference in HbA1c between SMBG and rtCGM (adjusted difference −15.3 mmol/mol; 95% CI, −21.5 to −9.1), with less pronounced differences seen with other ethnicities.
Interpretation
Inequities in CGM use exist based on prioritised ethnicity and socioeconomic status. Importantly, CGM was independently associated with lower HbA1c, suggesting that lack of CGM funding contributes to health disparity in children with T1D.
Funding
Australasian Paediatric Endocrine Group (APEG), Canterbury Medical Research Foundation, Starship Foundation.
Keywords: Continuous glucose monitoring, Type 1 diabetes, Paediatrics, Equity, Disparity, Diabetes technology
Research in context.
Evidence before this study
Authors BJW and MdB published a systematic review and meta-analysis which provide evidence for continuous glucose monitoring (CGM) improving glycaemic outcomes in type 1 diabetes (T1D). A search of the databases PubMed, Web of Science, Scopus, and Cochrane Central register of clinical trials was conducted up to April 2021. Studies not in English language were excluded, but no other filters were applied. The complete search terms list used were as follows: (‘type 1 diabetes’ OR t1d OR ‘insulin dependent diabetes’ OR iddm) AND (‘continuous glucose monitor∗’ OR ‘flash glucose monitor∗’ OR ‘continuous subcutaneous glucose’ OR ‘glucose sensor’ OR ‘glucose-sensor’ OR cgm OR rtcgm OR fgm OR icgm OR iscgm OR ‘diabetes technology’ OR ‘sensor-guided’ OR ‘sensor guided’ OR ‘sensor augmented’ OR ‘sensor-augmented’ OR sap). The systematic review included 22 randomised controlled trials assessing differences in glycaemic outcomes between CGM and self-monitoring of blood glucose (SMBG) in T1D. The meta-analysis found CGM was beneficial for impacting glycaemic outcomes including glycated haemoglobin (HbA1c), time in range (TIR; 3.9–10 mmol/L [70–180 mg/dL]), and time below range (TBR; <3.9 mmol/L [<70 mg/dL]). Furthermore, newer non-adjunctive CGM technology produced the greatest improvement in TIR (6.0%; 95% CI, 2.3 to 9.7).
A search of the PubMed database up to May 2021 identified three studies which show unequal access to publicly funded insulin pump therapy in New Zealand according to ethnicity, socioeconomic status, and geographic location. However, no studies have previously evaluated access to non-funded CGM in New Zealand. Since the New Zealand health system does not directly fund or subsidise CGM the authors anticipated even greater disparities in access to CGM use, and based on the results of the meta-analysis the authors anticipated that unequal access would perpetuate existing inequity in glycaemic outcomes.
Added value of this study
This study provides the first insight into CGM use among children <15 years with T1D in New Zealand, confirming ethnic and socioeconomic inequities in access to CGM. The study builds on existing evidence that CGM is associated with healthier glycaemic control, and it shows this association is independent of potential confounding variables such as ethnicity and socioeconomic status. The results also show an interaction effect whereby the impact of CGM on HbA1c differs between ethnic groups, and universal use of CGM mitigates disparities in HbA1c based on ethnicity.
Implications of all the available evidence
The results of this study support studies reporting that uptake of other new diabetes technology differs by geographic location in New Zealand. It is important that paediatricians and paediatric endocrinologists are not gatekeepers to diabetes technologies. These results contrast studies which attribute inequities in health based on ethnicity to socioeconomic status since Māori children were found to have higher HbA1c and lower CGM use independent of socioeconomic status. These results add weight to the evidence that universal access to CGM for children with T1D is one method to address health inequity. Further, data from this study will allow for a true population impact if/when CGM becomes publicly funded in New Zealand.
Introduction
Several randomised controlled trials (RCTs) show continuous glucose monitoring (CGM) systems improving glycaemic outcomes, especially time in range (TIR) and reduced hypoglycaemia, for people with type 1 diabetes (T1D) regardless of age, mode of insulin delivery, glycaemic control, and impaired hypoglycaemic awareness.1 Real-world data now exists, confirming the positive impact of CGM on glycaemic outcomes2,3 and early adoption is associated with improved long-term outcomes.4 Accordingly, clinical guidelines for CGM use have been devised internationally,5,6 and diabetes guidelines recommend CGM is offered to all children and adults with T1D.7,8
CGM systems measure interstitial glucose concentration every 5 min (288 readings per day) and transmit graphical and numerical glucose data to a receiver or smartphone. Two main types of CGM are currently available; real-time (rtCGM) and intermittently scanned (isCGM).1 rtCGM automatically transmits continuous glucose data, providing real-time actionable information about current glucose level and trajectory. Comparatively, isCGM relies on manually scanning the sensor to obtain retrospective glucose data. rtCGM has been shown to produce higher TIR (3.9–10 mmol/L [70–180 mg/dL]) and less time below range (<3.9 mmol/L [<70 mg/dL]) compared to isCGM in head to head RCTs9, 10, 11 which is also supported in a meta-analysis.1 Furthermore, rtCGM is an essential component of automated insulin delivery (AID) systems which are superseding sensor augmented pump therapy in the management of T1D.12
As the evidence base for the clinical value of CGM has grown, rates of funding have also grown.13 Studies abroad, including Australia, have evaluated the real-world impact of universal CGM funding; reporting sharp increases in CGM uptake and reductions in glycated haemoglobin (HbA1c) following initiation of funding,14,15 as well as decreased hospitalisations, less vocational absenteeism and improved quality of life.15 Cost-effective analyses also favour rtCGM over self-monitoring of blood glucose (SMBG) and isCGM.16,17 Unlike many other countries of similar economic strength, CGM systems are not funded in Aotearoa/New Zealand (henceforth referred to as New Zealand), either publicly, or through health insurance. This is a concern since disparities in access to publicly funded insulin pump therapy already exist in New Zealand,18,19 and the high financial cost of self-funding CGM may perpetuate existing inequities in access to diabetes technology. Equitable access to advanced diabetes technologies, including CGM, is important because evidence suggests equal access can ameliorate the association between socioeconomic status and glycaemia.20 This is relevant to New Zealand where Māori, Pacific, and socioeconomically deprived children affected by T1D are at greater risk of unhealthy metabolic control.21
This study aims to provide data for national CGM use in children and youth affected by T1D in New Zealand, in order to understand the impact of a health service (public and/or through private health insurance) that does not subsidise or fully fund CGM.
Methods
A cross-sectional (descriptive and analytical) population-based study collected diabetes data (participant demographics, diabetes treatment, HbA1c and CGM use) from all regional diabetes centres in New Zealand with children <15 years affected by T1D as of 1st October 2021. The age cut-off reflects clinical practice in paediatric diabetes services in New Zealand.
Inclusion criteria included: children/adolescents aged <15 years, T1D diagnosed as per the American Diabetes Association, and under a secondary care paediatric diabetes service in New Zealand.
In New Zealand, an estimated >99% of children with T1D are managed by secondary care diabetes services.22 There are 20 district health boards (DHBs) across New Zealand (Supplemental Appendix Fig. 1) that are responsible for the provision of secondary care health services in their region. There are two instances where a single diabetes service covers more than one DHB: Auckland, Waitemata, and Counties Manukau DHBs; and Canterbury and West Coasts DHBs. All insulin and glucose test strips are fully funded in New Zealand, and publicly funded insulin pump therapy is available to individuals who meet pre-specified criteria: either those experiencing severe unexplained hypoglycaemia, or those with an HbA1c that is 65–90 mmol/mol (8.1–10.4%).23 CGM is not funded in New Zealand, and AID systems were not commercially available at the time of this study.
There is no national database for recording diabetes treatment and outcomes in New Zealand, so all 20 DHBs completed an Excel spreadsheet supplied by the research team. Data were obtained either from existing local databases, or established through electronic chart review in centres who do not collect data. All participant identifiers were removed prior to data-sharing and analysis. Data collected for each participant is shown in Supplemental Table 1.
Socioeconomic status was ascertained indirectly from address of domicile, coded to small area units (meshblocks, about the size of a city block), which were matched to the New Zealand Index of Deprivation 2018 (NZDep). NZDep, a validated area-based measure of socioeconomic position in New Zealand, combines the following census data: communication, income, employment, qualifications, owned home, support, living space, and living condition.24 NZDep is displayed as deciles with each decile containing about 10% of the New Zealand population.24 Decile 1 represents areas with the least socioeconomically deprived scores, and decile 10 represents areas with the most socioeconomically deprived scores. NZDep data were categorised into quintiles for analysis where quintile 5 represents the 20% most socioeconomically deprived areas in New Zealand based on meshblocks.
Ethnicity data were based on self-reported ethnicity data supplied by DHBs. Ethnicity data were analysed using a standardised prioritised system as follows: if multiple ethnicities were recorded, the participant was assigned to a single ethnicity by an established hierarchical classification of Māori (Indigenous People of New Zealand), Pacific, Asian, and then New Zealand European/Other, as per Ethnicity Data Protocols.25 The New Zealand European/Other category was almost exclusively New Zealand Europeans (805 European, 22 Middle Eastern/Latin American/African (MELAA), and 21 ‘Other’).
Health outcome measurement was based on HbA1c with the last recorded HbA1c in the prior six months up to 1st October 2021. Glucose management indicator (GMI) was not used if a point of care/local laboratory HbA1c test was not available. HbA1c data were not included in the analysis for participants (n = 92) diagnosed with T1D for less than six months.
CGM use was categorised into rtCGM and isCGM. The frequency of CGM use was not collected as these data are unreliably recorded. For those using CGM, access was categorised as self-funded, supplied via a research study, or supplied via income assistance programs (Work and Income New Zealand).
The audit activity of this study was covered by “Clinical benchmarking utilising data from New Zealand Diabetes Centre Patient Management Systems/Databases and contributing to the Australasian Diabetes Database Network/SWEET international diabetes database”, Ethics Committee reference number HD18/098.
Statistical methods
Demographic and clinical characteristics of the study sample were summarised using standard descriptive measures (counts and percentages for categorical variables, and medians and interquartile ranges [IQR] for continuous variables). CGM use (including both isCGM and rtCGM) was summarised as counts and percentages according to children's characteristics. Associations between CGM use and children's characteristics were tested using univariable and multivariable generalised linear regression models followed by asymptotic chi-square tests. Pairwise group comparisons were calculated as risk ratios (RR) with 95% confidence intervals (CI), using Poisson regression models with robust ‘sandwich’ standard errors.
Factors associated with participants’ glycaemic control were investigated by using linear regression models to estimate between-group differences in mean HbA1c with 95% CI. The importance of access to CGM in driving observed differences was assessed by adjusting univariable estimates first by glucose monitoring modality (SMBG, isCGM, or rtCGM) alone, and then in a multivariable model including all factors of interest.
P values from hypothesis tests were adjusted using the Benjamini & Hochberg procedure for controlling the false discovery rate, and 95% CI from regression models were adjusted using a modified Dunnett method. Analysis was conducted using R Statistical Software (v4.1.1; R Core Team 2021), with the package emmeans (v1.7.0; Lenth 2021) used to estimate marginal means and group contrasts.
Role of the funding source
Funding sources for the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Results
Description of sample population
Data were supplied for 1209 eligible children with T1D from all 20 DHBs. A description of the study sample is shown in Supplemental Table 2. The majority of children with T1D fall within the 10 – <15 years age bracket (63.2%) and identify as European/Other ethnicity (70.2%), followed by Māori (18.1%), Pacific (7.1%), and Asian (4.6%). Secondary care diabetes centres varied in participant number from 8 (0.7% of the New Zealand cohort) to 291 (24.1% of the New Zealand cohort). Median HbA1c (IQR) was 64 (56–75) mmol/mol [8.0%]. Injections were the commonest mode of insulin delivery (57.7%) with the remaining 40.0% using insulin pump therapy, and only 2.3% were using AID. Most AID use was within the context of research since AID systems were not commercially available in New Zealand on 1st October 2021. CGM was used by 67.5% of the study population with the majority of these using isCGM (59.7%) over rtCGM (40.3%).
CGM use
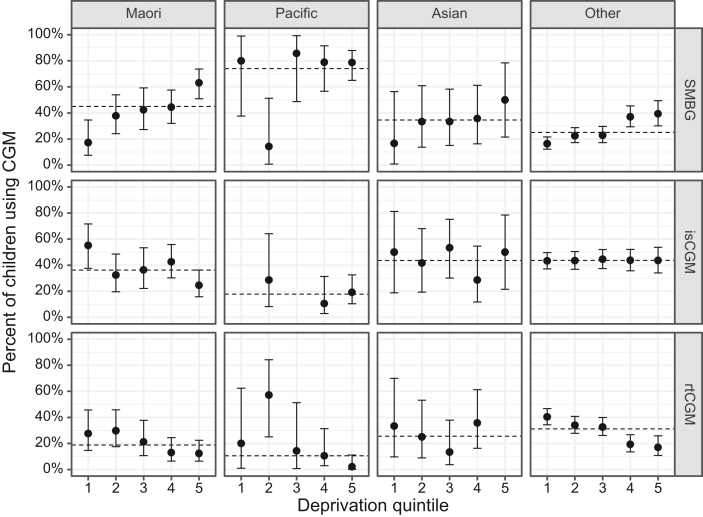
Unadjusted associations between CGM use (both rtCGM and isCGM) and socio-demographic variables are presented in Table 1. Lower unadjusted rates of CGM use were associated with: increasing age, with an unadjusted RR for the 10 to <15 years age band of 0.80 (95% CI, 0.70 to 0.93); Māori and Pacific ethnicities (Fig. 1); higher socioeconomic deprivation (Fig. 1); Counties Manukau DHB (unadjusted RR 0.57; 95% CI, 0.40 to 0.81) (Supplemental Fig. 2); and insulin injections.
Table 1.
Associations between demographic and clinical factors with use of CGM.
| Demographic characteristic | Total n (%, n = 1205) | Uses CGM n (row %) | Unadjusted risk ratio (95% CI) | Adjusted risk ratio (95% CI) |
|---|---|---|---|---|
| Age – no. (%) | P = 0·002 | P = 0·013 | ||
| <5 yr | 72 (6·0%) | 58 (80·6%) | 1 | 1 |
| 5 to < 10 yr | 370 (30·7%) | 261 (70·5%) | 0·88 (0·75, 1·02) | 0·94 (0·81, 1·09) |
| 10 to < 15 yr | 763 (63·3%) | 494 (64·7%) | 0·80 (0·70, 0·93) | 0·85 (0·73, 0·99) |
| Gender – no. (%) | P = 0·50 | P = 0·99 | ||
| Female | 604 (50·1%) | 414 (68·5%) | 1 | 1 |
| Male | 601 (49·9%) | 399 (66·4%) | 0·97 (0·90, 1·05) | 1·00 (0·93, 1·07) |
| Ethnicity – no. (%) | n = 1204 | P < 0·001 | P < 0·001 | |
| Māori | 218 (18·1%) | 120 (55·0%) | 1 | 1 |
| Pacific | 85 (7·1%) | 22 (25·9%) | 0·47 (0·30, 0·74) | 0·62 (0·40, 0·96) |
| Asian | 55 (4·6%) | 36 (65·5%) | 1·19 (0·91, 1·56) | 1·24 (0·96, 1·60) |
| European/Other | 846 (70·3%) | 634 (74·9%) | 1·36 (1·17, 1·58) | 1·20 (1·04, 1·38) |
| New Zealand Deprivation Index (quintile) – no. (%) | n = 1203 | P < 0·001 | P < 0·001 | |
| 1 (least deprived) | 279 (23·2%) | 230 (82·4%) | 1 | 1 |
| 2 | 256 (21·3%) | 192 (75·0%) | 0·91 (0·81, 1·02) | 0·92 (0·83, 1·03) |
| 3 | 230 (19·1%) | 165 (71·7%) | 0·87 (0·77, 0·98) | 0·91 (0·81, 1·02) |
| 4 | 224 (18·6%) | 130 (58·0%) | 0·70 (0·60, 0·82) | 0·79 (0·68, 0·93) |
| 5 (most deprived) | 214 (17·8%) | 95 (44·4%) | 0·54 (0·44, 0·66) | 0·69 (0·57, 0·84) |
| Diabetes information | ||||
| Duration of diagnosis, mean (SD) | n = 1203 | P = 0·81 | P = 0·12 | |
| <2 yr | 361 (30·0%) | 242 (67·0%) | 1 | 1 |
| 2 to < 4 yr | 285 (23·7%) | 189 (66·3%) | 0·99 (0·87, 1·12) | 0·91 (0·81, 1·02) |
| 4 to < 15 yr | 557 (46·3%) | 380 (68·2%) | 1·02 (0·92, 1·13) | 0·90 (0·80, 1·01) |
| District Health Board – no. (%) | P < 0·001 | P < 0·001 | ||
| Northland | 43 (3·6%) | 26 (60·5%) | 0·88 (0·60, 1·28) | 0·97 (0·69, 1·38) |
| Waitemata | 102 (8·5%) | 60 (58·8%) | 0·85 (0·66, 1·11) | 0·85 (0·67, 1·07) |
| Auckland | 74 (6·1%) | 43 (58·1%) | 0·84 (0·62, 1·14) | 0·87 (0·65, 1·16) |
| Counties Manukau | 115 (9·5%) | 46 (40·0%) | 0·57 (0·40, 0·81) | 0·70 (0·51, 0·96) |
| Waikato | 108 (9·0%) | 75 (69·4%) | 1·02 (0·83, 1·25) | 1·07 (0·88, 1·30) |
| Bay of Plenty | 59 (4·9%) | 48 (81·4%) | 1·20 (0·98, 1·47) | 1·09 (0·89, 1·34) |
| Taranaki | 43 (3·6%) | 28 (65·1%) | 0·95 (0·67, 1·34) | 0·92 (0·63, 1·33) |
| Lakes | 31 (2·6%) | 21 (67·7%) | 0·99 (0·68, 1·45) | 1·13 (0·78, 1·62) |
| Tairawhiti | 12 (1·0%) | 8 (66·7%) | 0·97 (0·52, 1·81) | 1·24 (0·60, 2·55) |
| Whanganui | 23 (1·9%) | 13 (56·5%) | 0·82 (0·47, 1·43) | 1·02 (0·57, 1·84) |
| Mid Central | 55 (4·6%) | 34 (61·8%) | 0·90 (0·65, 1·25) | 0·92 (0·66, 1·27) |
| Hawkes Bay | 53 (4·4%) | 33 (62·3%) | 0·91 (0·65, 1·26) | 1·00 (0·75, 1·34) |
| Capital and Coast | 69 (5·7%) | 50 (72·5%) | 1·06 (0·84, 1·34) | 0·98 (0·80, 1·20) |
| Hutt Valley | 63 (5·2%) | 46 (73·0%) | 1·07 (0·84, 1·36) | 1·00 (0·81, 1·25) |
| Wairarapa | 8 (0·7%) | 8 (100·0%) | 1·49 (1·38, 1·61) | 1·29 (1·03, 1·60) |
| Nelson/Marlborough | 38 (3·2%) | 29 (76·3%) | 1·12 (0·85, 1·49) | 0·97 (0·76, 1·24) |
| West Coast | 13 (1·1%) | 12 (92·3%) | 1·37 (1·06, 1·76) | 1·30 (0·93, 1·81) |
| Canterbury | 150 (12·4%) | 117 (78·0%) | 1·15 (0·99, 1·33) | 1·09 (0·95, 1·26) |
| South Canterbury | 35 (2·9%) | 25 (71·4%) | 1·05 (0·75, 1·46) | 0·81 (0·59, 1·11) |
| Southern | 111 (9·2%) | 91 (82·0%) | 1·21 (1·04, 1·41) | 1·02 (0·88, 1·18) |
| Insulin modality – no. (%) | P < 0·001 | P < 0·001 | ||
| Injections | 693 (57·5%) | 372 (53·7%) | 1 | 1 |
| Insulin pump | 512 (42·5%) | 441 (86·1%) | 1·60 (1·49, 1·73) | 1·48 (1·36, 1·61) |
∗ Adjusted risk ratios are adjusted for age, gender, ethnicity, deprivation, duration of diabetes, district health board (DHB), and insulin modality.
∗∗ The reference category for DHB is all ‘other DHBs in New Zealand’.
∗∗∗ Waitemata, Auckland and Counties Manukau are three DHBs under a single regional diabetes service, and Canterbury and West Coast are two DHBs under a single regional diabetes service.
∗∗∗∗ Wald P values calculated using finite sample F statistic and adjusted for multiplicity using the false discovery rate (FDR). Confidence intervals are adjusted using the Bonferroni method for DHB and Dunnett method for all other comparisons.
∗∗∗∗∗ Insulin pump therapy includes both standard pump therapy and sensor augmented pump therapy.
Fig. 1.
Percent of children using glucose monitoring modality by ethnicity and socioeconomic deprivation. Point range plots showing unadjusted percentages (95% binomial confidence intervals) of children <15 years with T1D according to glucose monitoring modality (rows), prioritised ethnicity (columns), and New Zealand Deprivation (NZDep) quintile where quintile 1 is the least deprived and quintile 5 is the most deprived. SMBG is self-monitoring of blood glucose, isCGM is intermittently scanned CGM, and rtCGM is real-time CGM. Dashed horizontal lines represent overall means for each ethnicity.
Adjusted associations between CGM use and socio-demographic variables are presented in Table 1. After adjustment (for age, gender, ethnicity, NZDep, duration of diabetes, DHB, and insulin modality), those of European/Other ethnicity were 20% more likely to be utilising CGM compared to Māori (adjusted RR 1.20; 95% CI, 1.04 to 1.38), and Pacific children were 38% less likely to be utilising CGM compared to Māori (adjusted RR 0.62; 95% CI, 0.40 to 0.96). Increasing deprivation was inversely associated with CGM use with NZDep quintile 5 using CGM 31% less than quintile 1 (adjusted RR 0.69; 95% CI, 0.57 to 0.84). Older age was also associated with less CGM use (adjusted RR 0.85; 95% CI, 0.73 to 0.99). Differences in CGM use by DHB persisted after adjustment for subject level factors (P < 0.001), with use remaining low in Counties Manukau DHB compared with the rest of New Zealand.
Impact of CGM use on HbA1c
The effect of CGM use on HbA1c was found to differ by ethnicity, so HbA1c outcomes based on ethnicity are stratified by CGM use (Table 2). No such interaction effect was found with other variables, including NZDep, so for these variables CGM is adjusted for as a potential confounder (Supplemental Table 3).
Table 2.
Associations between ethnicity and CGM use with level of glycaemic control.
| Glucose monitoring modality and ethnicity | HbA1c mmol/mol |
HbA1c difference mmol/mol (comparator group - reference group) |
|
|---|---|---|---|
| Mean (SD) | Unadjusted difference (95% CI) | Fully adjusted difference (95% CI) | |
| SMBG | |||
| Māori (n = 88) | 83·5 (19·3) | 0 | 0 |
| Pacific (n = 55) | 78·4 (18·8) | −5·0 (−10·9, 0·8) | −5·0 (−10·9, 0·9) |
| Asian (n = 16) | 63·2 (13·0) | −20·2 (−29·4, −11·0) | −16·5 (−25·6, −7·5) |
| European/Other (n = 182) | 68·7 (16·3) | −14·7 (−19·1, −10·3) | −12·1 (−16·5, −7·7) |
| isCGM | |||
| Māori (n = 71) | 71·9 (16·8) | 0 | 0 |
| Pacific (n = 13) | 75·5 (15·4) | 3·5 (−6·7, 13·8) | 0·7 (−9·4, 10·8) |
| Asian (n = 22) | 65·6 (14·8) | −6·3 (−14·6, 2·0) | −8·5 (−16·5, −0·4) |
| European/Other (n = 343) | 64·1 (13·2) | −7·8 (−12·2, −3·4) | −7·2 (−11·5, −2·8) |
| rtCGM | |||
| Māori (n = 40) | 61·8 (10·9) | 0 | 0 |
| Pacific (n = 9) | 69·9 (14·2) | 8·1 (−4·4, 20·7) | 7·8 (−4·4, 20·1) |
| Asian (n = 11) | 55·2 (9·4) | −6·6 (−18·1, 5·0) | −5·0 (−16·3, 6·3) |
| European/Other (n = 242) | 59·3 (10·5) | −2·4 (−8·2, 3·4) | −1·6 (−7·3, 4·1) |
∗ Ethnicity and CGM use were associated with HbA1c in unadjusted and adjusted models (P < 0·001). Ethnicity was found to be an effect modifier on CGM use (unadjusted P = 0·002, adjusted P = 0·013); therefore, results are presented stratified by CGM use. For all other variables please see Supplementary Table 3.
∗∗ Fully adjusted HbA1c is adjusted for age, gender, ethnicity, deprivation, duration of diabetes, district health board, insulin modality, and CGM use.
∗∗∗ SMBG is self-monitoring of blood glucose, isCGM is intermittently scanned CGM, and rtCGM is real-time CGM.
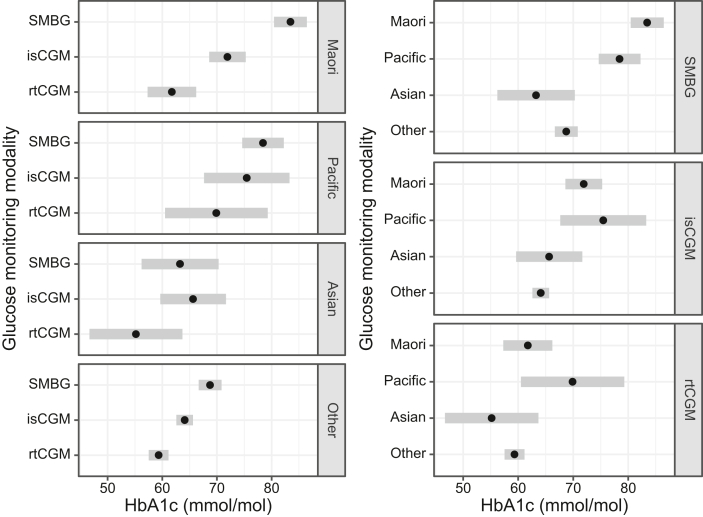
Mean HbA1c levels were lowest for children utilising rtCGM (Table 2 and Fig. 2). For these children there was no evidence of a difference in HbA1c by ethnicity, but numbers of Asian and Pacific children utilising rtCGM were too small to rule out potentially large differences. Mean HbA1c was higher for children utilising isCGM and higher still for children utilising SMBG. Differences in HbA1c between children utilising SMBG versus rtCGM was most marked for children of Māori ethnicity (adjusted difference −15.3 mmol/mol; 95% CI, −21.5 to −9.1). Among children utilising SMBG, mean HbA1c levels were clearly lower for children of Asian or European/Other ethnicity, but disparities in HbA1c between ethnic groups were less pronounced among children utilising CGM (Fig. 2). Among children utilising SMBG, the European/Other group had a mean HbA1c 12.1 mmol/mol lower (95% CI, −16.5 to −7.7) and the Asian group had a mean HbA1c 16.5 mmol/mol lower (95% CI, −25.6 to −7.5) than Māori children. However, among children utilising rtCGM, the European/Other group had a mean HbA1c 1.6 mmol/mol lower (95% CI, −7.3 to 4.1) and the Asian group 5.0 mmol/mol lower (95% CI, −16.3 to 6.3) than children identifying as Māori.
Fig. 2.
HbA1c by ethnicity, stratified by CGM use. Point range plots showing the estimated mean HbA1c (mmol/mol) of children with type 1 diabetes in New Zealand, according to their ethnicity and continuous glucose monitoring (CGM) use. SMBG is self-monitoring of blood glucose, isCGM is intermittently scanned CGM, and rtCGM is real-time CGM. Black dots with grey bands represent estimated mean HbA1c with 95% confidence intervals. Within each panel, pairwise comparisons of groups with confidence intervals that do not overlap may be considered to differ with P < 0·05.
Unadjusted, CGM adjusted and fully adjusted associations between demographic/clinical factors and HbA1c are presented in Supplemental Table 3. Lower HbA1c was associated with: decreasing age; decreasing NZDep quintile; decreasing duration of diabetes; Canterbury DHB; and insulin pump therapy following adjustment in the multivariable model. However, HbA1c was not associated with gender. Children in NZDep quintile 5 had an HbA1c 5.2 mmol/mol higher than children in quintile 1, children with a duration of diabetes 4–15 years had an HbA1c 4.8 mmol/mol higher than those diagnosed for <2 years, and multiple daily injections was associated with an HbA1c 4.9 mmol/mol higher than insulin pump therapy. Differences in HbA1c between DHBs also persisted following adjustment with Canterbury DHB being associated with an HbA1c 6.0 mmol/mol lower than the rest of New Zealand.
Discussion
Equitable access to diabetes technologies is essential to health equity, especially given that certain demographic groups already experience a higher burden of diabetes and its complications.21 This national data set identifies compelling health inequities based on ethnicity for children with T1D in New Zealand.
This study disputes the notion that inequities relating to ethnicity in T1D outcomes are socioeconomically driven,26 since children with T1D of European/Other ethnicity were privileged with lower HbA1c compared to Māori and Pacific children, independent of socioeconomic deprivation. This is an important finding as this implies in addition to socioeconomic status, ethnicity-based treatment bias and/or unique ethnicity or cultural factors lead to disparities in diabetes health outcomes. Further, the study highlights that disparities in HbA1c based on ethnicity are mitigated by the use of rtCGM since there was very little difference in HbA1c between Māori and European children utilising rtCGM regardless of adjustment or not. Additionally, Māori children showed the greatest difference in HbA1c between SMBG and rtCGM, suggesting they are set to gain the greatest benefit from universal CGM funding in New Zealand.
Children of European/Other ethnicity were privileged with 20% more CGM use than Māori children, and Pacific children had even lower rates of CGM use (38% lower than Māori) independent of socioeconomic status. Systemic inequities in access to CGM based on ethnicity are anticipated to be even greater among adults with T1D in New Zealand because all children with T1D receive the Child Disability Allowance; a non-means tested allowance which families often use to support financing CGM (there is only a small gap between this allowance and the cost of isCGM, and therefore reflects the dominant choice of CGM in New Zealand). Despite equal access to the Child Disability Allowance, CGM use was also strongly associated with socioeconomic status: those from the most deprived socioeconomic backgrounds affected by T1D were 31% less likely to be using CGM compared to those from the least deprived socioeconomic backgrounds.
Lower rates of CGM use are described among minoritized ethnicities and socioeconomically deprived individuals in countries with funded CGM.27 In New Zealand, disparities in access to publicly funded insulin pump therapy exist according to DHB.18 The present study also supports that geographic location independently influences diabetes technology utilisation in New Zealand, with lower rates of CGM use in Counties Manukau DHB. While residual confounding is a plausible explanation for observed rates in Counties Manukau DHB (high proportion of minoritized ethnicities and socioeconomic deprivation), these findings suggest system-level barriers related to financial cost and funding are not solely accountable for disparities in technology use, and compounding provider-level barriers may be responsible. Engagement of Māori families when seeking support for their children within the New Zealand health system has been shown to be influenced by the effects of institutionalised racism. Factors such as socioeconomic deprivation, other barriers to access, and interpersonal and internalised racism were all cited as reasons for difficulties engaging.28
The results of this study provide a formidable argument for rtCGM to be publicly funded in New Zealand, and New Zealand should learn from its insulin pump example, which illustrates that access criteria can amplify health inequities.18 Initiation of publicly funded CGM is only the first step however. In addition, there is a need for health policy and strategy to specifically support enhanced access to diabetes technologies for Māori that is appropriate for their needs in order to improve diabetes outcomes. The Pae Ora (Healthy Futures) Bill ensures the Māori Health Authority will be an equal partner alongside the new entity, Health NZ in Health Systems Reform,29 and it is hoped that inequity in access to CGM will be addressed within this process. Further, paediatricians and paediatric endocrinologists who are important gatekeepers to diabetes treatments,30 should be included in targeted interventions and adequately resourced to support Māori to access and sustain diabetes technologies. Co-design of the New Zealand health system to facilitate health equity for Māori with T1D is the forthcoming challenge.
Strengths of this study include: the study sample, comprising all 20 DHBs estimated to represent >99% of all children <15 years with T1D in New Zealand, makes sampling bias unlikely and the findings more reliable; HbA1c was excluded from the analysis for participants diagnosed for less than six months since HbA1c is not a reliable marker of glycaemia in this context; the study collected relevant socio-demographic (ethnicity and NZDep) and CGM (CGM type and funding) data; and the little missing HbA1c data for children diagnosed for more than six months (n = 19) was similar between CGM and SMBG groups.
The study has several limitations including an inability to make a causal inference (the cross-sectional nature does not preclude the possibility that participants with lower HbA1c were more likely to adopt CGM), but our findings are consistent with randomised controlled trials showing improved glycaemic control in CGM intervention groups.1 The study design did not account for participants who recently stopped using CGM (such as an inability to continue funding it, or device intolerance), and binary data on CGM use was collected (‘yes’ or ‘no’) rather than clarifying if participants met recommended CGM data sufficiency criteria.5 The data is also limited by the accuracy of data collected by DHBs since no secondary data validation was undertaken by the researchers. Whilst this study aimed to prioritise ethnicity as per standardised protocols, it is acknowledged that with the vast majority of participants identifying as one ethnicity, self-prioritisation may have been requested by health administration staff at point of collection.25 Further, numbers of children using rtCGM were low for Pacific and Asian ethnic groups, limiting the ability to show superiority to isCGM; insulin modality did not distinguish between standard pump therapy or sensor augmented pump therapy; and NZDep is an area-based measure of socioeconomic deprivation with the potential for residual confounding at the individual level.
This study confirms the presence of inequities in access to CGM for children <15 years affected by T1D in New Zealand, a health system that does not directly fund or subsidise this technology. Furthermore, the study builds on existing evidence that CGM is associated with healthier glycaemic control. These results add to the evidence that universal access to CGM for children affected by T1D is one way to address health outcome inequity in T1D for children in New Zealand.
Contributors
MdB, BJW, CJ, EW, and RP were the lead clinical investigators providing supervision and acquiring funding for the project. MB administered the project. MdB, MB, BJW, EW, RP, CJ, JW, and YA contributed to the methodology. MB, HD, YA collected data. MB, HD, and JW curated data. MB and JW undertook the formal statistical analysis. MB wrote the original draft, and all authors critically reviewed the manuscript and contributed to the interpretation of the results. MdB, MB, and JW are the guarantors of this work and, as such, had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The study protocol is available as supplementary material. There are strong privacy laws in New Zealand, and the ethics waiver of consent restricts the sharing of this data to the Australasian Diabetes Database Network and SWEET international diabetes database.
Declaration of interests
MB has received research grants from the Health Research Council of New Zealand and Australasian Paediatric Endocrine Group. HD has no conflicts of interest to declare. CJ, RP, BW, and EW are named investigators and MdB is a named Principal Investigator on the Australasian Paediatric Endocrine Group grant provided for this study. RP has received research grants from the Health Research Council of New Zealand, Lotteries, Cure Kids, Royal New Zealand College of General Practitioners, Waikato Medical Research Foundation, and University of Waikato. RP has received educational honoraria from Eli Lilly, Boehringer Ingelheim, Novo Nordisk, Astra Zeneca, Sanofi, Novartis, and Blue Horizons. RP is on the Eli Lilly New Zealand Medical Advisory Board. RP is an Executive Member of the New Zealand Society for the Study of Diabetes, Ministry of Health Expert Diabetes Advisory Group Member, Royal Australasian College of Physicians Adult General Medicine Committee member, and Convener of the New Zealand Diabetes Guidance Group. RP was previously president of the New Zealand Society of Endocrinology. BW has received research grants and equipment from Medtronic, Dexcom, and iSENS. JW is a named investigator on research grants received from the Health Research Council of New Zealand, Canterbury Medical Research Foundation, Dexcom, Otago Medical Research Foundation, National Science Challenge, the Rea Charitable Trust. JW has had research contracts with the Ministry of Health, New Zealand, and the Health Quality and Safety Commission, New Zealand with payments made to his employer/institution, the University of Otago. YA is Chairperson of the Tamariki Pakari Child Health and Wellbeing Trust. MdB has received research grants from Dexcom and Medtronic, and research equipment from Dexcom, Medtronic, and Ypsomed. MdB has received honoraria from Medtronic, Ypsomed, and Dexcom.
Acknowledgments
This work was funded by an Australasian Paediatric Endocrine Group (APEG), with additional funding from the Canterbury Medical Research Foundation, and Starship Foundation. The researchers would also like to thank the DHB representatives who kindly collected data: Erin Bedford, South Canterbury DHB; Gilli Lewis, Sarah Diamond, and Rebecca Stern, Capital and Coast DHB; Rita Sigley, Auckland DHB; Anita Lala, Bay of Plenty DHB; Chris Davidson, Hawkes Bay DHB; Lucy Bish, Hutt Valley and Wairarapa DHBs; Jaco Nel, Lakes DHB; Nigel Orr, Mid-central DHB; Vicki Cunningham, Northland DHB; Stanley Ng, Tairawhiti DHB; Yiing Goh, Waikato DHB; Mitchelle Lagera, Taranaki DHB; Daniel Hiley, Canterbury DHB; Pauline Giles, Whanganui DHB.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2022.100644.
Appendix A. Supplementary data
References
- 1.Elbalshy M., Haszard J., Smith H., et al. Effect of divergent continuous glucose monitoring technologies on glycaemic control in type 1 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. Diabet Med. 2022 doi: 10.1111/dme.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardona-Hernandez R., Schwandt A., Alkandari H., et al. Glycemic outcome associated with insulin pump and glucose sensor use in children and adolescents with type 1 diabetes. Data from the international pediatric registry SWEET. Diabetes Care. 2021;44(5):1176–1184. doi: 10.2337/dc20-1674. [DOI] [PubMed] [Google Scholar]
- 3.Champakanath A., Akturk H.K., Alonso G.T., Snell-Bergeon J.K., Shah V.N. Continuous glucose monitoring initiation within first year of type 1 diabetes diagnosis is associated with improved glycemic outcomes: 7-year follow-up study. Diabetes Care. 2022;45(3):750–753. doi: 10.2337/dc21-2004. [DOI] [PubMed] [Google Scholar]
- 4.Prahalad P., Ding V.Y., Zaharieva D.P., et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: the pilot 4T study. J Clin Endocrinol Metab. 2021;107(4):998–1008. doi: 10.1210/clinem/dgab859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danne T., Nimri R., Battelino T., et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battelino T., Danne T., Bergenstal R.M., et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMeglio L.A., Acerini C.L., Codner E., et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(S27):105–114. doi: 10.1111/pedi.12737. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence . 2022. Diabetes (type 1 and type 2) in children and young people: diagnosis and management (NG18) Last updated 31 March 2022. [PubMed] [Google Scholar]
- 9.Hásková A., Radovnická L., Petruželková L., et al. Real-time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: the CORRIDA randomized controlled trial. Diabetes Care. 2020;43(11):2744–2750. doi: 10.2337/dc20-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy M., Jugnee N., El Laboudi A., Spanudakis E., Anantharaja S., Oliver N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med. 2018;35(4):483–490. doi: 10.1111/dme.13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visser M.M., Charleer S., Fieuws S., et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397(10291):2275–2283. doi: 10.1016/S0140-6736(21)00789-3. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S85–S99. doi: 10.2337/dc21-S007. [DOI] [PubMed] [Google Scholar]
- 13.Graham C. Continuous glucose monitoring and global reimbursement: an update. Diabetes Technol Therapeut. 2017;19(S3):S60–S66. doi: 10.1089/dia.2017.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson S.R., Holmes-Walker D.J., Chee M., et al. Universal subsidized continuous glucose monitoring funding for young people with type 1 diabetes: uptake and outcomes over 2 Years, a population-based study. Diabetes Care. 2022;45(2):391–397. doi: 10.2337/dc21-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charleer S., Mathieu C., Nobels F., et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real-world study. J Clin Endocrinol Metab. 2018;103(3):1224–1232. doi: 10.1210/jc.2017-02498. [DOI] [PubMed] [Google Scholar]
- 16.Pease A., Zomer E., Liew D., Lo C., Earnest A., Zoungas S. Cost-effectiveness of health technologies in adults with type 1 diabetes: a systematic review and narrative synthesis. Syst Rev. 2020;9(1):171. doi: 10.1186/s13643-020-01373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isitt J.J., Roze S., Tilden D., et al. Long-term cost-effectiveness of Dexcom G6 real-time continuous glucose monitoring system in people with type 1 diabetes in Australia. Diabet Med. 2022 doi: 10.1111/dme.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler B.J., Braund R., Galland B., et al. District health board of residence, ethnicity and socioeconomic status all impact publicly funded insulin pump uptake in New Zealand participants with type 1 diabetes. N Z Med J. 2019;132(1491):78–89. [PubMed] [Google Scholar]
- 19.Hennessy L.D., De Lange M., Wiltshire E.J., Jefferies C., Wheeler B.J. Youth and non-European ethnicity are associated with increased loss of publicly funded insulin pump access in New Zealand people with type 1 diabetes. Diabet Med. 2021;38(1) doi: 10.1111/dme.14450. [DOI] [PubMed] [Google Scholar]
- 20.Addala A., Auzanneau M., Miller K., et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2020;44(1):133–140. doi: 10.2337/dc20-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter P.J., Cutfield W.S., Hofman P.L., et al. Ethnicity and social deprivation independently influence metabolic control in children with type 1 diabetes. Diabetologia. 2008;51(10):1835. doi: 10.1007/s00125-008-1106-9. [DOI] [PubMed] [Google Scholar]
- 22.de Bock M., Jones T.W., Fairchild J., Mouat F., Jefferies C. Children and adolescents with type 1 diabetes in Australasia: an online survey of model of care, workforce and outcomes. J Paediatr Child Health. 2019;55(1):82–86. doi: 10.1111/jpc.14122. [DOI] [PubMed] [Google Scholar]
- 23.PHARMAC. Pharmaceutical Schedule [Internet]. [cited Nov 2022] 2022. https://schedule.pharmac.govt.nz/2022/11/01/Schedule.pdf# Available from:
- 24.Atkinson J., Salmond C., Crampton P. University of Otago; Wellington: 2019. NZDep2018 Index of Deprivation, Interim Research Report, December 2019.https://www.otago.ac.nz/wellington/otago730394.pdf Available from: [Google Scholar]
- 25.Ministry of Health . Ministry of Health; Wellington: 2017. HISO 10001:2017 Ethnicity Data Protocols. [Google Scholar]
- 26.Nazroo J.Y. The structuring of ethnic inequalities in health: economic position, racial discrimination, and racism. Am J Public Health. 2003;93(2):277–284. doi: 10.2105/ajph.93.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong J.C., Foster N.C., Maahs D.M., et al. Real-time continuous glucose monitoring among participants in the t1d exchange clinic registry.(Report) Diabetes Care. 2014;37(10):2702. doi: 10.2337/dc14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild C.E.K., Rawiri N.T., Willing E.J., Hofman P.L., Anderson Y.C. What affects programme engagement for Māori families? A qualitative study of a family-based, multidisciplinary healthy lifestyle programme for children and adolescents. J Paediatr Child Health. 2021;57(5):670–676. doi: 10.1111/jpc.15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.New Zealand Government . New Zealand Government; 2022. New beginning for Health System: Pae Ora (Healthy Futures) Bill passes third reading.https://www.beehive.govt.nz/release/new-beginning-health-system-pae-ora-healthy-futures-bill-passes-third-reading Available from: [Google Scholar]
- 30.Walker A.F., Hood K.K., Gurka M.J., et al. Barriers to technology use and Endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44(7):1480. doi: 10.2337/dc20-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.