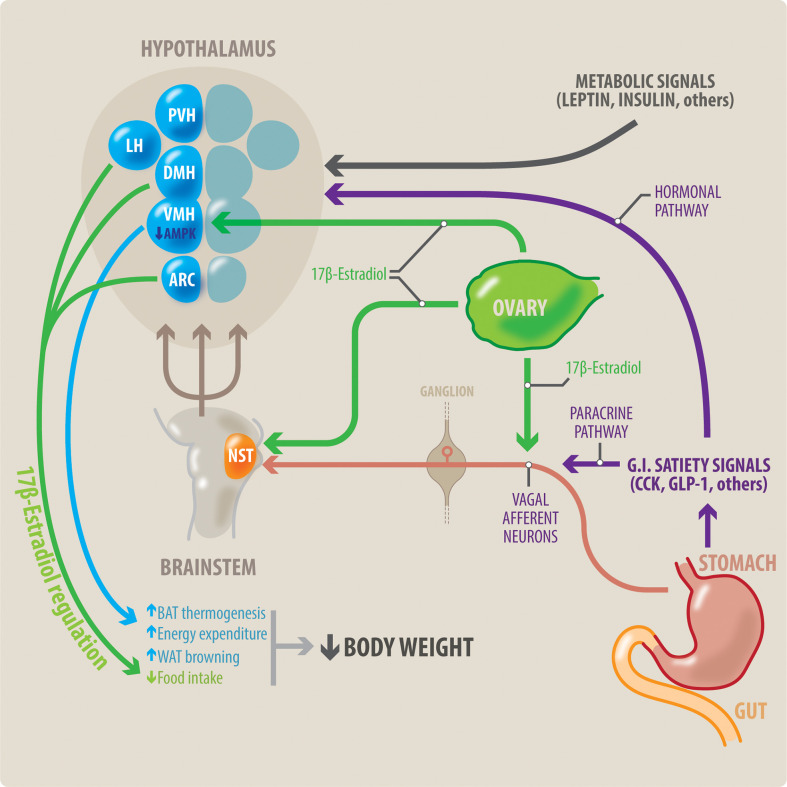
Figure 1.
Potential interaction between meal-related gastrointestinal signals and estradiol on control of the body weight in women. Meal-related gastrointestinal signals (CCK, GLP-1, others) act through a paracrine-neuronal pathway (shown in purple and red). These meal-related gastrointestinal signals act paracrinally upon vagal afferent neurons (VAN). The VANs activate secondary neurons located in the NTS, in the brainstem. The NTS integrates a variety of peripheral signals, and in turn activates tertiary neurons located in different nuclei in the hypothalamus. These hypothalamic nuclei control feeding behavior. Circulating estradiol (shown in green) modulates the responsiveness to these gastrointestinal satiety signals by acting on all levels of this paracrine-neuronal pathway: The VAN, NTS and the hypothalamic nuclei. Gastrointestinal satiety signals also act directly upon the hypothalamic nuclei through a hormonal pathway (shown in purple). Additionally, estradiol (green) has a direct anorexigenic effect at the level of the hypothalamic nuclei (PVH, LH and ARC), thereby reducing food intake. Metabolic signals such as insulin and leptin also influence centers in the hypothalamus to regulate body weight. Brown adipose tissue (BAT) thermogenesis contributes to regulation of body weight by increasing energy expenditure. Estradiol acts all three points of the VMH-SNS-BAT pathway to increase thermogenesis. Within the VMH hypothalamic nucleus, estradiol acts by inhibiting AMPK. Thus, estradiol increases energy expenditure by increasing BAT thermogenesis, and WAT browning. This, in combination with estradiol’s effects to decrease food intake, can result in weight loss. NST, nucleus of the solitary tract; DMH, dorsomedial hypothalamus; LH, lateral hypothalamus; PVH, paraventricular hypothalamus; ARC, arcuate nucleus; VMH, ventromedial hypothalamus; BAT, brown adipose tissue; WAT, white adipose tissue.