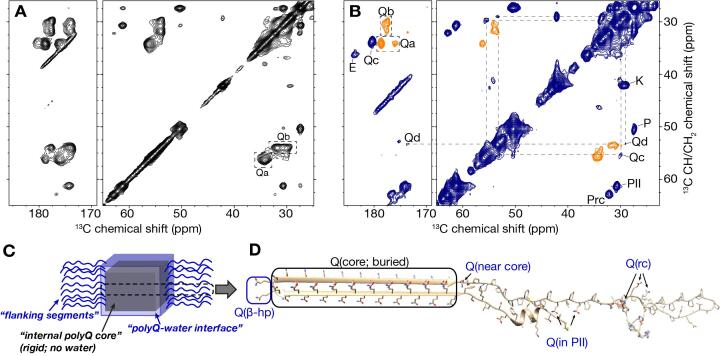
Fig. 7.
Intermediate-motion selection DYSE in 13C–13C 2D MAS NMR. (A) 2D 13C–13C DARR experiment at 277 K. The dominant Cα-C β /Cγ peaks of the rigid polyQ core signals “Qa” and “Qb” are marked with dashed boxes (right). (B) Corresponding DIPSHIFT-filtered DARR experiment at 277 K, using a 0.20 ms R1817 dipolar recoupling period at 10 kHz MAS. The previously marked rigid core signals now are negative (orange), while previously invisible glutamine signals become visible as positive peaks (blue). These are marked as Qc and Qd. Other strong positive signals from non-Gln residues outside the fiber core are also marked (E, K, P residues). The amino acid type assignment and secondary structure analysis was done using PLUQ program (Fritzsching et al., 2016, 2013). (C) Schematic drawing of the fibril structure, distinguishing the internal fiber core (i.e. dry interfaces; black) from segments and residues outside the core (wet interface; flanking domains; blue). (D) Schematic monomer in our model of the Q44-HttEx1 fibril structure, marking the locations of glutamine residues outside the dry fiber core: β-turn residues labelled as Q(β-hp), terminal polyQ segment residues (“near core”), and a few Gln in the dynamic PRD indicated as “rc” and “PII”. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)