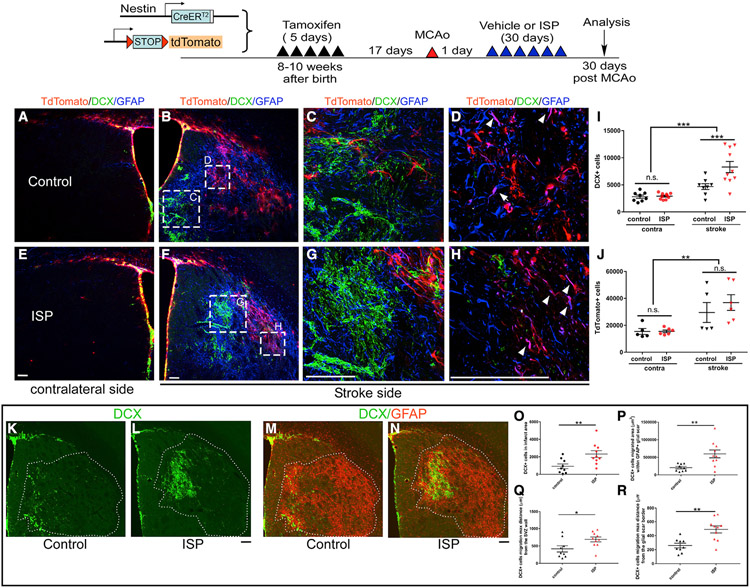
Figure 3. ISP treatment increased newly born neuroblast migration deep into the striatum 1 month after stroke.
For lineage tracing, Nestin creER-tdTomato mice were used. Stroke mice receiving vehicle (A–D, K, and M) or ISP treatment (E–H, L, and N) starting from day 1 post-stroke for 30 days. Contralateral side showing minimal migration of DCX+ neuroblasts (arrows) or tdTomato+ cells into the striatum (A, E, I, and J). Stroke strongly enhances the migration of newly born astrocytes (double-positive for tdTomato and GFAP, arrows in D and H; for single channel images, see Figure S4), but disallows the migration of DCX+ neuroblasts deeply into the lesion (B–D, I, and J). ISP treatment enhances DCX+ neuroblast migration (G, I, L, and N) but did not change total tdTomato+ cells (F, H, I, and J). ISP treatment enhanced the total number of DCX+ cells that penetrated into the glial scarred lesion area at 30 days post-stroke (K–O) and the migration of DCX+ into the glial scarred lesion area: (P)total DCX+ cell migrated area (Q) furthest distance migrated from the lateral wall of ventricle and (R) furthest distance migrated from the border of the glia scar in striatum. **p < 0.01 and ***p < 0.001, ANOVA for (I and J) and Student’s t test for (O–R). Each data point represents the average of 1 animal (average for each animal is obtained by quantifying multiple brain sections expanding the stroke infarct volume). Data were combined from 2 independent cohorts of mice.