Abstract
Introduction
Anti-LDL receptor-related protein 2 (anti-LRP2) nephropathy is a rare but progressive form of autoimmune-mediated tubulointerstitial nephritis and glomerular disease, characterized by a classic pattern of immune complex deposition in the kidney. A theoretic link between autoimmune disease and lymphoproliferative diseases exists, and therefore, in some cases autoimmune-mediated inflammation and lymphoproliferative neoplasm can co-exist in the same site.
Case Presentation
An elderly man presented with 6 months of weight loss and fatigue. Subsequent workup showed an elevated serum creatinine and subnephrotic range proteinuria. Kidney biopsy was performed which revealed anti-LRP2 nephropathy with concurrent primary kidney extranodal marginal zone lymphoma. He was subsequently treated with rituximab but remains dialysis-dependent (12 months after his initial diagnosis, at time of publication of this report).
Conclusion
We discuss the bidirectional relationship between autoimmune disease and lymphoma in the kidney, along with a brief review of the literature pertaining to these rare lesions. Our case report highlights the diagnostic difficulties faced by pathologists when encountering this entity. We also attempt to spread awareness about the co-existence of tubulointerstitial inflammation and lymphoproliferative disorder, which may be under-recognized.
Keywords: Anti-LDL receptor-related protein 2 nephropathy, Tubulointerstitial nephritis, Anti-brush border antibody, Megalin, Proximal tubule, Extranodal marginal zone lymphoma
Introduction
Anti-LDL receptor-related protein 2 (anti-LRP2) nephropathy, also called anti-brush border antibody disease, is a rare autoimmune kidney disease with fewer than 50 cases reported in the literature to date [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. The disease is characterized by peripheral circulating antibodies to LRP2, also known as megalin [4]. Patients are typically >65 years of age and often present with acute kidney injury (AKI) with or without proteinuria. The few reported cases of anti-LRP2 nephropathy suggest that it is a progressive disease, with patients often progressing to end-stage kidney disease shortly after diagnosis. On kidney biopsy, it has a very characteristic pattern of immune complex-mediated tubulointerstitial nephritis, with polytypic IgG deposits seen along proximal tubular basement membranes and Bowman's capsule. Glomerular involvement is also common but is usually limited to segmental membranous-type deposits along peripheral capillary walls. The term “anti-brush border” antibody disease comes from the location of the target antigen LRP2 (megalin) which is found on proximal tubular brush borders. Anti-LRP2 nephropathy has been reported to occur de novo, or in association with other autoimmune diseases and malignancies [1, 2, 3, 4, 5, 9]. We hereby report an unusual case of anti-LRP2 nephropathy, diagnosed concurrently in the same biopsy with extranodal marginal zone lymphoma.
Case Report
Initial Presentation
The patient was a 79-year-old man who presented with 6 months of weight loss and decreased energy. Leading up to his presentation, he had lost 16 lbs (7.3 kg) over 6 months, despite having a good appetite. He had a past medical history of hypertension, asthma, and benign prostatic hypertrophy. There was no history of fevers, night sweats, rashes, and arthralgias. There was no known family history of kidney disease. His medications included simvastatin, mometasone, cetirizine, albuterol inhaler, and budesonide/formoterol.
He was found to have an increase in his serum creatinine from a baseline of 1.1 mg/dL 2 years ago to 5.9 mg/dL during his most recent office visit. He was also found to have anemia, with a hemoglobin of 7.7 g/dL and new onset subnephrotic range proteinuria with a urine protein-to-creatinine ratio of 1.9 g/g creatinine. On his most recent laboratory studies, his serum albumin was 2.5 g/dL. He was nonoliguric, producing 1.0–1.5 L of urine daily. His routine serologic workup including hepatitis B, hepatitis C, HIV, ANA, ANCA, and anti-GBM antibodies were all unremarkable. His complement levels were normal. His urinalysis showed glucosuria (250 mg/dL). Urine microscopy showed 3–5 RBCs/HPF and 11–20 WBC/HPF. Overall, his serum and urine studies were concerning for a proximal tubule defect, given his hypokalemia (3.3 mmol/L), normal anion gap metabolic acidosis (serum bicarbonate of 21 mmol/L), glucosuria (250 mg/dL on urinalysis with a serum glucose of 105 mg/dL), and proteinuria. His serum protein electrophoresis revealed 2 restricted bands migrating in the gamma region. Serum free light chains showed elevated kappa light chains measured at 1,657 mg/L, lambda light chains at 22 mg/L, and a kappa/lambda ratio of 75. Kidney ultrasound showed that he had no hydronephrosis bilaterally, with his right and left kidneys measuring 11.1 cm and 11.9 cm, respectively.
Therefore, a kidney biopsy was performed for further evaluation of the AKI and subnephrotic range proteinuria. Due to his age and abnormal light chain studies, there was a high suspicion for paraprotein-related kidney disease.
Kidney Biopsy Findings
Light Microscopy
The kidney biopsy demonstrated 42 glomeruli, 18 of which were globally sclerotic. The background tubulointerstitium showed moderate to severe interstitial fibrosis and tubular atrophy. The nonatrophic cortex showed interstitial inflammation, with a mixed inflammatory infiltrate comprising numerous reactive plasma cells, lymphocytes, monocytes, and scattered eosinophils (shown in Fig. 1, top left). Scattered focal tubulitis and acute tubular injury were seen (minor component). In addition to this, some areas of the infiltrate had an atypical appearance composed of monomorphic lymphoid cells with hyperchromatic nuclei and scant cytoplasm. Glomeruli were relatively unremarkable, albeit for mild ischemic changes. Arteries and arterioles showed moderate intimal fibrosis and luminal narrowing. No atypical casts or crystal deposition was noted.
Fig. 1.
Top left: diffuse tubulointerstitial inflammation, with some areas of the infiltrate showing morphological atypia in the form of hyperchromasia (right half of field) (H&E stain, ×100 magnification; scale bar, 50 μm). Top right: granular staining of proximal tubular basement membranes, Bowman's capsule, and segmental glomerular capillary walls (IgG IF stain, ×200 magnification; scale bar, 50 μm). Bottom left: small electron-dense immune complexes seen along proximal tubular basement membrane (transmission EM, approx. ×4,800 magnification; scale bar, 2 μm). Bottom right: small electron-dense immune complexes seen in subepithelial locations (membranous-type) and along Bowman's capsule (transmission EM, approx. ×2,900 magnification; scale bar, 4 μm). IF, immunofluorescence; EM, electron microscopy.
Immunofluorescence Microscopy and Immunohistochemical Stains
Immunofluorescence (IF) stains revealed a unique pattern of tubulointerstitial immune complex deposition (shown in Fig. 1, top right). Deposits were seen along proximal tubular basement membranes and along Bowman's capsule. Some glomeruli also showed segmental granular peripheral capillary wall staining. The glomerular and tubular basement membrane deposits showed IgG (2+), C3 (2+), kappa (2+), and lambda (1+) staining. Kappa showed a mild increase in background staining. C1q was negative. Patchy brush border staining was also seen with IgG. IgG subclass staining revealed a polytypic pattern to both glomerular and tubulointerstitial deposits (IgG1: 2+, IgG2: 0, IgG3: 0, and IgG4: 1+). Phospholipase A2 receptor was negative. Due to the unusual pattern of tubulointerstitial immune complex deposition, anti-LRP2 nephropathy was suspected, and therefore, LRP2 IF staining on formalin-fixed paraffin-embedded tissue was requested (EMD Millipore, Billerica, MA, USA: performed at Arkana Laboratories, Little Rock, AR, USA). The stains revealed positivity for LRP2 in the tubular basement membrane and Bowman's capsule deposits (shown in Fig. 2). Glomerular deposits were negative for LRP2. Immunohistochemical (IHC) staining for SV40 (polyomavirus) was negative. IgG4 IHC staining revealed only rare IgG4-positive plasma cells. In retrospect, changes suggestive of glomerular and tubular basement membrane deposits were not readily visible by light microscopy.
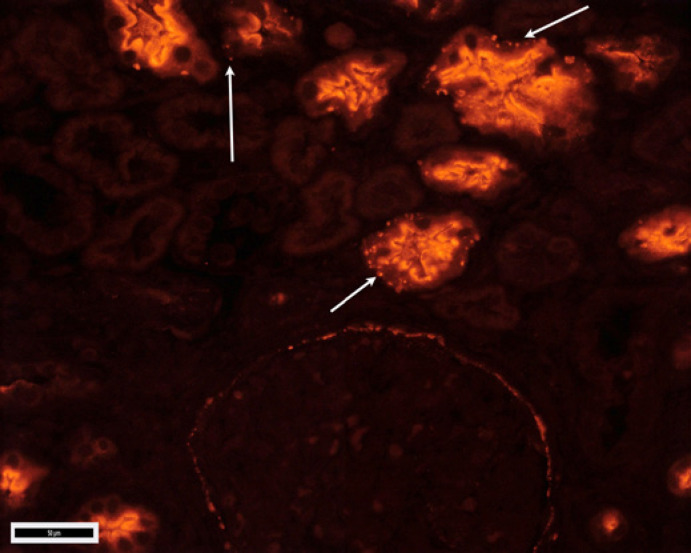
Fig. 2.
Granular staining of proximal tubular basement membranes, Bowman's capsule, and segmental glomerular capillary walls; corresponding to IgG stain (LRP2 IF stain, ×200 magnification; scale bar, 50 μm; image courtesy of Dr. Tiffany Caza, Arkana Laboratories, Little Rock, AR, USA). IF, immunofluorescence; LRP2, LDL receptor related protein 2
Electron Microscopy
Electron microscopy confirmed the presence of electron-dense deposits along tubular basement membranes (shown in Fig. 1, bottom left), Bowman's capsule, and segmentally in glomerular subepithelial locations (shown in Fig. 1, bottom right). The deposits did not show substructure. No finely granular deposits or fibrillary or other organized forms of deposits were identified. Glomeruli were otherwise unremarkable, except for podocyte foot process effacement in areas of subepithelial immune complex deposition.
IHC and Molecular Testing Performed to Characterize the Atypical Lymphoid Infiltrate
The atypical lymphoid cells showed expression of CD20 (diffuse), PAX5 (diffuse), CD10 (subset), and BCL2 (shown in Fig. 3). BCL6 was positive in a small subset of the neoplastic B-cells, and BCL1 was negative. CD43 was also expressed in a small subset of the neoplastic cells. CD5 and CD21 were negative. CD3 highlighted reactive T cells. The neoplastic cells also showed staining with kappa, while lambda was negative (in situ hybridization). Fluorescence in situ hybridization studies for BCL2, BCL6, and MALT1 gene rearrangements were negative. B-cell clonality studies on the tissue revealed clonal rearrangements in the heavy and kappa light chain immunoglobulin loci (IgH and IgK), consistent with B-cell clonality. Based on the morphological appearance and immunophenotypic findings, including positivity for CD43, BCL6, and aberrant CD10 expression in a subset of the cells, a diagnosis of lymphoplasmacytic lymphoma was considered less likely. MYD88 testing could not be performed due to lack of tissue. Therefore, the overall morphological, IHC, and molecular features were thought to be most consistent with a diagnosis of extranodal marginal zone lymphoma.
Fig. 3.
Neoplastic lymphoid infiltrate showing plasmacytoid features, including small, round, and eccentrically located nuclei with condensed chromatin and reduced cytoplasm. (H&E stain, ×400 magnification; scale bar, 50 μm). Inset CD3 (top) and CD20 (bottom).
Diagnosis and Post-Biopsy Course
Based on the clinical presentation and biopsy findings, the patient was diagnosed as having anti-LRP2 nephropathy. Subsequent radiological workup for systemic lymphadenopathy or masses was negative, and he was therefore diagnosed with concurrent primary extranodal marginal zone lymphoma of the kidney. He received 4 doses of rituximab at 375 mg/m2 per week for treatment of both his lymphoma and autoimmune disease. Twelve months after his initial diagnosis, he continues to remain dialysis-dependent. Follow-up on trends of hematological parameters was not available.
Discussion
Our case report highlights a unique association between anti-LRP2 nephropathy and concurrent lymphomatous infiltration of the kidney. This is a rare association but has been previously described. In 2019, Gamayo et al. [1] described 2 similar cases of anti-LRP2 nephropathy and concurrent low-grade B-cell lymphoma involving the kidney. Among these, 1 case had lymphoplasmacytic lymphoma and the other had chronic lymphocytic leukemia. Our case of anti-LRP2 nephropathy with extranodal marginal zone lymphoma is similar in that a low-grade B-cell lymphoma was concurrently found based on the suspicious clinical features (abnormal serum protein electrophoresis) and the subsequent investigation of a morphologically atypical population of lymphoid cells in the biopsy. All 3 cases showed similar morphological features to the tubulointerstitial nephritis (TIN), showed polytypic IgG staining to the immune complexes, had confirmatory anti-LRP2 staining, and revealed no other forms of paraprotein-related kidney disease (Table 1).
Table 1.
Cases of anti-LRP2 nephropathy concurrently diagnosed with lymphoma
| Author | Age/sex | Histological findings | IgG subclass distribution of deposits | Type of lymphoma | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Gamayo et al. [1] | 74/M | Immune complex-mediated TIN with moderate chronicity | IgG4>IgG1>IgG2>Ig3 | LPL | Bortezomib, dexamethasone, and rituximab | Dialysis-dependent in <1 month |
|
| ||||||
| Gamayo et al. [1] | 70/M | Immune complex-mediated TIN with moderate chronicity | IgG1»Ig2/IgG4 | CLL | Rituximab | 2 months of follow-up: mild improvement in sCr |
|
|
||||||
| IgG3 negative | ||||||
|
| ||||||
| Ng et al. (current report) | 79/M | Immune complex-mediated TIN with moderate chronicity | IgG1»IgG4 | EMZL | Rituximab | 7 months of follow-up: dialysis-dependent |
|
|
||||||
| IgG2 and IgG3 negative | ||||||
LPL, lymphoplasmacytic lymphoma; CLL, chronic lymphocytic leukemia; EMZL, extranodal marginal zone lymphoma; LRP2, LDL receptor related protein 2; TIN, tubulointerstitial nephritis.
Anti-LRP2 nephropathy itself may be a very under-recognized disease, and hence, recent awareness of the entity may allow for its subsequent increased detection. Indeed, 18 of the 20 or so cases in the renal pathology literature have been reported in the last 24 months. Anti-LRP2 nephropathy should therefore be included in the differential diagnosis for all cases of immune complex-mediated TIN, which also includes lupus nephritis, IgG4-related disease, drug-induced TIN, polyomavirus nephropathy, and idiopathic hypocomplementemic TIN. Dinesh et al. [3] reported a case of anti-LRP2 with prominent IgG4-positive plasma cells, therefore identifying a unique diagnostic pitfall in distinguishing between entities in the differential diagnosis mentioned earlier (i.e.,: IgG4-related TIN, TIN in lupus nephritis, and anti-LRP2 nephropathy − all of which may have prominent IgG4-positive plasma cells). Therefore, although further studies are needed, anti-LRP2 nephropathy could be added to the list of kidney diseases with increased interstitial IgG4-positive plasma cells (which also includes ANCA-associated vasculitis). The characteristic distribution pattern of the immune complexes in anti-LRP2 nephropathy, namely, proximal tubular basement membrane, Bowman's capsule, and segmental glomerular capillary wall, should be the clue that leads the pathologist to the diagnosis. If available, confirmatory LRP2 staining can be considered, although this is not necessary in pathologically and clinically classic cases. Neoplastic lymphoid cells may be sparse, nonmass-forming, and possibly even present in perirenal tissues [3]. Therefore, there should be a very low threshold for initiating a lymphoma workup in these cases. Any morphologically atypical or monomorphic lymphoid population should be interrogated immunohistochemically, with at least a limited panel of CD3 and CD20. In our case, the initial IF stains revealed a slight increase in background staining with kappa; and in retrospect, this was another subtle clue to the elusive clonal lymphoid population in the biopsy. From a clinical perspective, one should consider anti-LRP2 nephropathy if an older patient presents with AKI, has signs of proximal tubular dysfunction on laboratory studies, and has a kidney biopsy with a protracted tubular injury pattern with associated tubular basement membrane and proximal tubular brush border IgG deposits. If tissue staining cannot be done, anti-LRP2 antibodies can be checked for serologically (not performed in our case). Many of the previously reported cases of anti-LRP2 nephropathy have been discovered in the setting of other autoimmune diseases, most commonly systemic lupus erythematosus [4, 5]. In addition to the anti-LRP2 nephropathy cases with concurrent lymphoma, at least 1 case of anti-LRP2 nephropathy has been reported in the setting of an underlying solid organ malignancy (renal cell carcinoma) [9]. Therefore, if anti-LRP2 nephropathy is confirmed histologically, one may consider checking for signs and symptoms of other autoimmune diseases and/or malignancy. Age-appropriate cancer screening may also be considered, if clinically relevant.
There appears to be a bidirectional relationship between autoimmune diseases and hematological malignancies, and the link between the two has been studied extensively. A cohort study of 878,161 patients with autoimmune disease found that individuals with certain autoimmune diseases, such as rheumatoid arthritis, autoimmune hemolytic anemia, celiac disease, immune thrombocytopenic purpura, Sjogren's syndrome, systemic lupus erythematosus, and polymyositis, had a higher standardized incidence ratio of non-Hodgkin's lymphoma [11]. Reasons for this are beyond the scope of this article, but theoretical explanations for this link could be chronic immune stimulation and immune fatigue, autoimmunity leading to inhibition of lymphoid cell death/apoptosis and possible shared genetic and environmental conditions [12, 13]. Vice versa, autoimmune phenomena may have seen various types of lymphoma. In the kidney in particular, a dysregulated local immune system in the setting of pre-existing lymphoma could result in the formation of polyclonal antibodies to self-antigens, leading to peripheral or in situ immune complex deposition in the kidney and subsequent kidney injury. Due to the polytypic nature of the immune complex composition in anti-LRP2 nephropathy, anti-LRP2 antibodies in these cases are unlikely to be produced by the neoplastic cells themselves.
Recent reports have described forms of malignancy-associated membranous nephropathy that show IgG1-dominant/co-dominant staining and a segmental distribution to the glomerular capillary wall deposits [14, 15]. Mass spectrometry interrogation of these deposits has revealed an increased association with the novel membranous nephropathy autoantigen “nerve epidermal growth-like factor 1” (NELL1) [14]. Other common membranous nephropathy autoantigens, like phospholipase A2 receptor and thrombospondin 7A, are consistently negative in these cases. In a case series of consecutive “segmental” forms of membranous nephropathy, authors from a large academic center concluded that segmental membranous nephropathy is a rare variant of membranous nephropathy, often associated with malignancy, and may have a favorable renal prognosis. In their study, up to 29% of cases (17/50) showed NELL1 positivity by tissue staining [15]. Therefore, in anti-LRP2 nephropathy cases associated with a clinical history of malignancy (such as the current case), there is a possibility that the glomerular component of immune complex deposition is driven by the malignancy itself. This would also explain the discordance between the anti-LRP2 staining in the glomerular and tubulointerstitial deposits. Our case was also polytypic IgG1-dominant (both glomerular and tubulointerstitial deposits) similar to these reports of segmental membranous nephropathy. Unfortunately due to logistical reasons NELL1 and thrombospondin 7A tissue staining/serologic testing could not be performed on our case.
To conclude, our case report demonstrates a novel association of anti-LRP2 nephropathy and extranodal marginal zone lymphoma, and helps enrich the existing knowledge of this disease. Anti-LRP2 nephropathy is a rare disease with a relatively poorer prognosis, possibly due to an insidious onset with most diagnoses being made late in the course of the disease. A diagnosis of anti-LRP2 nephropathy should be considered in all cases of immune complex-mediated tubulointerstitial nephritis; and although diagnostically challenging, the distinct distribution pattern of deposits should lead the pathologist to this diagnosis. Any morphologically atypical appearing lymphoid populations in the biopsy should be further evaluated immunohistochemically for the presence of neoplasia. Anti-LRP2 nephropathy and primary kidney lymphoma are both exceedingly rare, and our report may help to further shed light on this unusual association [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 16].
Statement of Ethics
The subjects have given their written informed consent to publish their case (including publication of images). The study protocol was approved by the institute's committee on human research (IRB#18-26348).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was used for this study.
Author Contributions
Lauren Ng contributed to data collection and organization, manuscript writing, and final manuscript review. Roberto Ruiz-Cordero contributed to data collection and final manuscript review. Tiffany Caza contributed to data collection and final manuscript review. Vighnesh Walavalkar contributed to data collection and organization, manuscript writing, final manuscript review, and submission.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Funding Statement
No funding was used for this study.
References
- 1.Gamayo A, Hecox D, Dicker L, Vecchiarelli L, Raess PW, Khalighi M, et al. Anti-LRP2 nephropathy with concurrent kidney infiltration by lymphoma. Clin Kidney J. 2019 Dec 22;13((3)):468–472. doi: 10.1093/ckj/sfz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy J, Andeen N, Gougeon F, Palmer M, Larsen C. Anti-brush border antibody disease: a clinicopathologic analysis of 27 cases (abs1689). In “Abstracts from USCAP 2020: medical renal pathology (1662–1696)ˮ. Mod Pathol. 2020;33((Suppl 2)):1590. [Google Scholar]
- 3.Dinesh KP, Raniele D, Michels K, Avasare CP, Kayton R, Khalighi MA, et al. Avasare anti-LRP2 nephropathy with abundant IgG4-positive plasma cells: a case report. Am J Kidney Dis. 2019 Jul;74((1)):132–137. doi: 10.1053/j.ajkd.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 4.Larsen CP, Trivin-Avillach C, Coles P, Collins AB, Merchant M, Ma H, et al. LDL receptor-related protein 2 (megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol. 2018 Feb;29((2)):644–653. doi: 10.1681/ASN.2017060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvanajscak Z, Murphy JD, Larsen CP, Padala SA. Anti-brush border antibody disease (anti-LRP2 nephropathy) associated with lupus nephritis. Kidney Int Rep. 2020 Jul 3;5((9)):1590–1594. doi: 10.1016/j.ekir.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallan AJ, Garg A, Collins AB, Beck LH, Trivin-Avillach C, Henriksen KJ, et al. Anti-LRP2 nephropathy. Kidney Int Rep. 2020 Sep 12;5((12)):2365–2370. doi: 10.1016/j.ekir.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X, Tu L, Liu S, You H, Xue J, Hao C. Complete remission of nephrotic syndrome in a young woman with anti-LRP2 nephropathy after immunosuppressive therapy. BMC Nephrol. 2020 Aug 24;21((1)):364. doi: 10.1186/s12882-020-02027-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caliskan Y, Caza T, Mosman A, Elawa U, Philipneri M, Martin K, et al. A case of immune complex mediated tubulointerstitial disease and nephrotic syndrome: anti LRP-2 nephropathy with diffuse podocyte effacement. J Nephrol. 2021 Jun;34((3)):915–919. doi: 10.1007/s40620-020-00762-9. [DOI] [PubMed] [Google Scholar]
- 9.Campbell RE, Uhlenhopp D, Shaw M, Dai DF, Sekar A. Anti-brush border antibody (ABBA)-associated renal disease. QJM. 2020 Aug 1;113((8)):561–562. doi: 10.1093/qjmed/hcaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosales IA, Collins AB, do Carmo PA, Tolkoff-Rubin N, Smith RN, Colvin RB. Immune complex tubulointerstitial nephritis due to autoantibodies to the proximal tubule brush border. J Am Soc Nephrol. 2016 Feb;27((2)):380–384. doi: 10.1681/ASN.2015030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25((10)):2025–2030. doi: 10.1093/annonc/mdu365. [DOI] [PubMed] [Google Scholar]
- 12.Goldin LR, Landgren O. Autoimmunity and lymphomagenesis. Int J Cancer. 2009;124((7)):1497–502. doi: 10.1002/ijc.24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jardin F. Development of autoimmunity in lymphoma. Expert Rev Clin Immunol. 2008;4((2)):247–266. doi: 10.1586/1744666X.4.2.247. [DOI] [PubMed] [Google Scholar]
- 14.Caza TN, Hassen SI, Dvanajscak Z, Kuperman M, Edmondson R, Herzog C, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021 Apr;99((4)):967–976. doi: 10.1016/j.kint.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudose S, Santoriello D, Debiec H, Canetta PA, Bomback AS, Stokes MB, et al. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. 2021 Jan;99((1)):247–255. doi: 10.1016/j.kint.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Hu D, Fang L, Chen Y, Che X, Tao J, et al. Primary renal lymphoma: a case report and literature review. Oncol Lett. 2016;12((5)):4001–4008. doi: 10.3892/ol.2016.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.