Abstract
Oligometastatic non-small-cell lung cancer (NSCLC) is potentially curable. Oligo-recurrence occurs with oligometastatic disease characterized by well-controlled primary lesion. The purpose of the present study was to explore the value of definitive local therapy (DLT) for extracranial single-organ oligorecurrent NSCLC. A total of 81 patients with NSCLC who had extracranial single-organ oligorecurrence after receiving radical treatment at the Cancer Hospital of the University of Chinese Academy of Sciences from January 2010 to December 2017 were analyzed. The primary endpoint was progression-free survival (PFS), and the secondary endpoint was overall survival (OS). The median follow-up time of the 81 patients was 65.8 months. A total of 39 patients received DLT. A large proportion of patients who did not accept DLTs received specific tyrosine kinase inhibitors (TKIs). The results of multivariate analysis showed that DLT and specific TKI therapy were favorable prognostic factors significantly related to PFS. Further analysis showed that for patients without specific TKI therapy, DLT significantly improved PFS and the 5-year PFS rate. The 5-year OS rate also improved, but the improvement was not significant. For extracranial single-organ oligorecurrent NSCLC, PFS was significantly superior in patients receiving DLT. Among them, for the subgroup of patients who did not receive specific TKI therapy, DLT is expected to improve long-term prognostic outcomes.
Keywords: non-small-cell lung cancer, recurrence, radiotherapy, surgery
1. Introduction
Lung cancer is one of the most common malignant tumors. Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers, and nearly 40% of patients are already in advanced stages at the time of diagnosis.[1] Clinicians have gradually realized that advanced NSCLC cannot be generalized in diagnosis and treatment. In fact, Hellman and Weichselbaum believed that there is an intermediate stage between local growth and extensive metastasis and proposed the concept of oligometastasis as early as 1998.[2] It is generally believed that for NSCLC, the presence of a certain number of metastases in a confined organ could be regarded as oligometastasis. A systematic review by Ashworth et al suggested that the prognosis of patients with oligometastatic NSCLC differed widely. The 5-year overall survival (OS) rate varied from 8.3% to 86%, with a median of 5.9 to 52 months, and the median time to any progression was 4.5 to 23.7 months. Although half of the patients progressed within 12 months, long-term survival did exist.[3] In addition, some scholars have proposed that the control of the primary tumor is also important for patients with oligometastatic disease. Even if metastatic lesions are effectively treated by local therapy, poorly controlled primary lesions will further progress to more extensive metastases, leading to therapeutic failure.[4] Therefore, Yuzuru and Chang proposed the concept of oligorecurrence, which is different from oligometastasis in that patients with oligorecurrence have well-controlled primary lesions. The goals of treatment for patients with oligometastasis and those of patients with oligorecurrence are different. The goal of the former is still prolonging the survival time before effectively controlling the primary lesion, while that of the latter should be to cure the disease.[5] The strategies for treating patients with oligometastasis in some malignant tumors already have concrete evidence, such as resection of liver metastatic lesions for colorectal cancer.[6] However, there is no sufficient evidence for the treatment strategy for oligometastatic and oligorecurrent NSCLC patients. The purpose of the present study was to evaluate the value of definitive local therapy (DLT) for NSCLC patients with extracranial single-organ oligorecurrence.
2. Methods
2.1. Patient selection
-
1.
Inclusion criteria.
-
1.1
Stage I to III NSCLC patients received radical treatment (including complete resection, stereotactic body radiation therapy and radical radiotherapy) at the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) from January 2010 to December 2017. This study was approved by the Ethical Committee of Zhejiang Cancer Hospital (Approval number: IRB-2022-189) and conducted in accordance with the Declaration of Helsinki.
-
1.2
The patients were followed up in our hospital and diagnosed with single-organ oligorecurrence with no more than 3 recurrent lesions and without local-regional recurrence (including the primary tumor or bronchial stump, bilateral hilum, bilateral mediastinum and bilateral supraclavicular area).
-
1.1
-
2.
Exclusion criteria
-
2.1
Brain metastases.
-
2.2
Metachronous second primary lung cancer (MPLC).
-
2.3
History of any other malignant tumor within 5 years before radical treatment.
-
2.4
Incomplete treatment information after recurrence.
-
2.1
2.2. DLT
DLT was defined as complete resection of metastatic lesions; radiosurgery, including gamma knife, CyberKnife, and stereotactic body radiotherapy (SBRT); microwave ablation; seed implantation; and radiotherapy ≥ 50 Gy.
2.3. Follow-up and diagnosis
Patients were followed up by means of telephone or outpatient visits. The frequency and contents of follow-up were determined by the attending physicians according to the patients’ disease status and method of treatment. The conventional contents of outpatient follow-up included medical history, physical examination, hematologic examination, chest computed tomography (CT) and upper abdomen CT/B-scan ultrasonography, as well as brain magnetic resonance imaging, radionuclide bone imaging, and positron-emission tomography–CT when necessary.
The diagnoses of oligorecurrence and disease progression were based on imaging or histopathological evidence. The diagnosis of MPLC was defined by the recommendations of the American College of Chest Physicians.[7] Recurrent lesions with different histological types were diagnosed as MPLC. For patients with recurrent lesions with the same histological type, MPLC was diagnosed if either of the following conditions were met: the recurrence interval was ≥ 4 years; the recurrence interval was 2 to 4 years, and the patient was diagnosed with MPLC by at least 2 radiologists. The recurrence interval was defined as the time interval from the previous radical treatment to the diagnosis of oligorecurrence.
2.4. Definition of survival outcome and sensitive mutations
The primary endpoint of the present study was progression-free survival (PFS), and the secondary endpoint was overall survival (OS). The starting points of PFS and OS were the date when the patient was diagnosed with oligorecurrence, and the end points were as follows: PFS: the date of disease progression or death due to any cause; OS: the date of death due to any cause. Patients were recorded as censored if they did not have an end-point event at the last follow-up.
Epidermal growth factor receptor (EGFR) sensitive mutations are defined as Exon 19 Deletion mutation and Exon 21 L858R mutation, which were detected by next generation sequencing (NGS) or Polymerase Chain Reaction. Anaplastic lymphoma kinase (ALK) sensitive mutation is defined as ALK fusion mutation, which was detected by NGS, Polymerase Chain Reaction or Ventana-D5F3 immunohistochemistry (IHC). Specific tyrosine kinase inhibitor (TKI) therapy refers to patients who had sensitive EGFR/ALK mutations and received corresponding EGFR/ALK TKI.
2.5. Statistics
Statistics were performed and graphics were plotted using SPSS 25.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA) software. The survival outcomes were calculated by Kaplan–Meier analysis, and comparisons between groups were performed by the log-rank test. The survival rate was compared by the Z test. The median follow-up time was calculated by inverse Kaplan–Meier analysis. Univariate and multivariate analyses of prognostic factors were performed by Cox risk regression. In addition to DLT, factors with statistical significance in the univariate analysis were included in the multivariate analysis. A 2-side P value < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
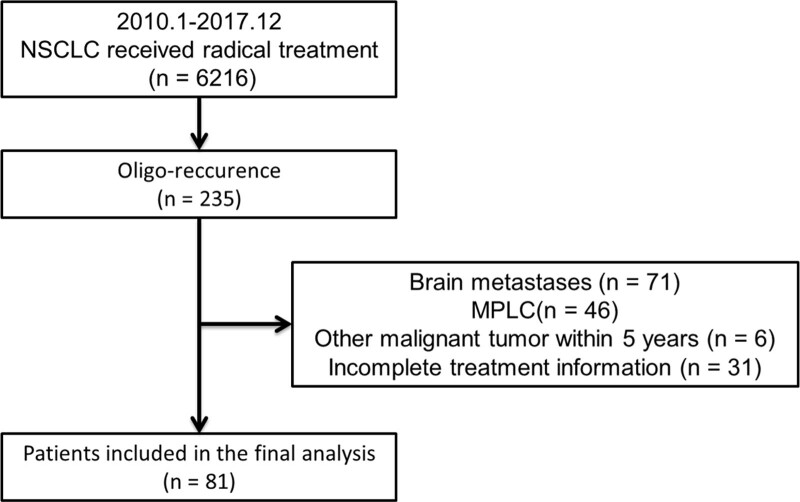
From January 2010 to December 2017, a total of 6216 Stage I to III NSCLC patients received radical treatment at the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital). A total of 81 patients were included in the study by preset inclusion and exclusion criteria. The screening process is shown in Figure 1. Patients included in the final analysis were 46 to 85 years old (median age 63 years). Previous radical treatments were as follows: 4 patients (4.9%) received SBRT, 65 patients (80.2%) received radical surgery, and 12 patients (14.8%) received radical radiotherapy. The sites of oligorecurrence included 30 (37.0%) with lung, 22 (27.2%) with bone, 12 (14.8%) with liver, 7 (8.6%) with adrenal gland, 5 (6.2%) with chest wall, 3 (3.7%) with kidney, and 2 (2.5%) with pleural nodules. Among the 81 patients, 39 patients received DLT. The baseline characteristics are presented in Table 1. For patients who did not receive DLT, patients with adenocarcinoma accounted for a greater proportion (P = .011), and more patients received specific TKI therapy (P < .001). There were no significant differences in the remaining baseline characteristics.
Figure 1.
Flowchart of patients selection. MPLC = metachronous second primary lung cancer.
Table 1.
Patients characteristics.
| Characteristics | No DLT (n = 42) | DLT (n = 39) | P value | |
|---|---|---|---|---|
| Gender | Male | 29 (69.0%) | 31 (79.5%) | .284 |
| Female | 13 (31.0%) | 8 (20.5%) | ||
| Age | <60 | 15 (35.7%) | 8 (20.5%) | .130 |
| ≥60 | 27 (64.3%) | 31 (79.5%) | ||
| Previous radical | Surgery | 35 (83.3%) | 30 (76.9%) | .822 |
| treatment | Radiotherapy | 5 (11.9%) | 7 (17.9%) | |
| SBRT | 2 (4.8%) | 2 (5.1%) | ||
| Initial TNM stage | I | 13 (31.0%) | 16 (41.0%) | .570 |
| II | 12 (28.6%) | 8 (20.5%) | ||
| III | 17 (40.5%) | 15 (38.5%) | ||
| Recurrent interval | <2 yrs | 34 (81.0%) | 32 (82.1%) | .899 |
| ≥2 yrs | 8 (19.0%) | 7 (17.9%) | ||
| ECOG PS | 0−1 | 41 (97.6%) | 36 (92.3%) | .556 |
| 2 | 1 (2.4%) | 3 (7.7%) | ||
| Number of lesions | 1 | 28 (66.7%) | 33 (84.6%) | .061 |
| 2−3 | 14 (33.3%) | 6 (15.4%) | ||
| Smoking history | No | 19 (45.2%) | 11 (28.2%) | .113 |
| Yes | 23 (54.8%) | 28 (71.8%) | ||
| Pathology | ADC | 28 (66.7%) | 15 (38.5%) | .011* |
| Others | 14 (33.3%) | 24 (61.5%) | ||
| Specific TKI therapy | No | 23 (54.8%) | 37 (94.9%) | P < .001* |
| Yes | 19 (45.2%) | 2 (5.1%) | ||
ADC = adenocarcinoma, ALK = anaplastic lymphoma kinase, DLT = definitive local therapy, ECOG PS = Eastern Cooperative Oncology Group Performance status, EGFR = epidermal growth factor receptor, SBRT = stereotactic body radiotherapy, TKI = tyrosine kinase inhibitor.
P < .05.
3.2. DLT
Of the 81 patients, 52 (64.2%) received local therapy for metastatic lesions, of which 39 (48.1%) received DLT. Twelve patients (30.8%) received complete surgical resection of metastatic lesions, 10 received SBRT (25.6%), 1 received CyberKnife (2.6%), 6 received microwave ablation (15.4%), 2 (5.1%) received seed implantation, and the remaining 8 patients (20.5%) received radiotherapy ≥ 50 Gy.
3.3. Oncogene testing and TKI treatment
Among the 81 patients, 51 (63.0%) patients received EGFR testing, of which 10 were tested by NGS, 1 by Cobas, and 40 by amplification refractory mutation system. For 43 adenocarcinoma patients, 41 (95.3%) received EGFR testing. A total of 21 patients were detected as harboring EGFR sensitive mutations, including 12 patients with Exon 19 Deletion and 9 patients with Exon 21 L858R. Fifty-five patients (67.9%) received ALK testing, of which 3 were tested by NGS, 1 by AMRS, and the remaining 51 were tested by Ventana-D5F3 IHC. For 43 adenocarcinoma patients, 33 (76.7%) received ALK testing. A total of 3 patients (6.0%) were ALK-positive, all detected by Ventana-D5F3 IHC. Therefore, a total of 24 patients had sensitive mutations, of which 21 received TKI, including 16 patients who received icotinib, 3 who received gefitinib, and 2 who received crizotinib.
3.4. Survival analysis
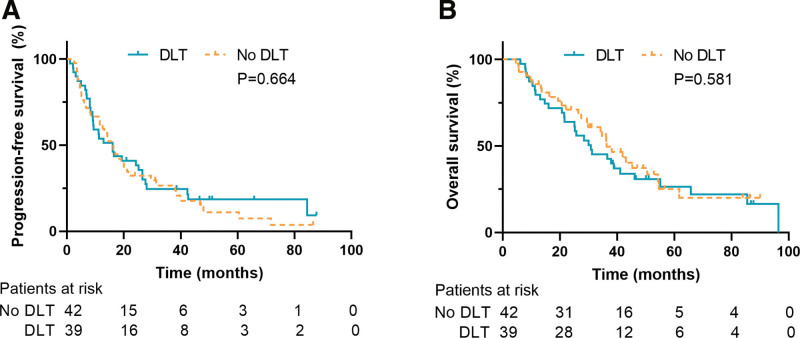
The median follow-up time of the 81 patients was 65.8 months, among which the those of the no DLT group and DLT group were 56.6 months and 65.8 months, respectively (P = .320). Fifty-eight patients had died at the time of data cutoff (28 in no DLT group and 30 in DLT group). Survival analysis (Fig. 2) indicated that the median PFS of the no DLT group was 16.2 months, and the 3-year and 5-year PFS rates were 26.6% and 11.1%, respectively. The median OS rate was 36.3 months, and the 3-year and 5-year OS rates were 55.3% and 25.2%, respectively. For the DLT group, the median PFS was 16.0 months, and the 3-year and 5-year PFS rates were 24.6% and 18.5%, respectively. The median OS was 30.8 months, and the 3-year and 5-year OS rates were 45.3% and 26.5%, respectively. The log-rank test indicated that there were no statistically significant differences in PFS (P = .664) or OS (P = .581) between the 2 groups.
Figure 2.
Survival outcomes of DLT group and No DLT group. (A) Progression-free survival. (B) Overall survival. DLT = definitive local therapy.
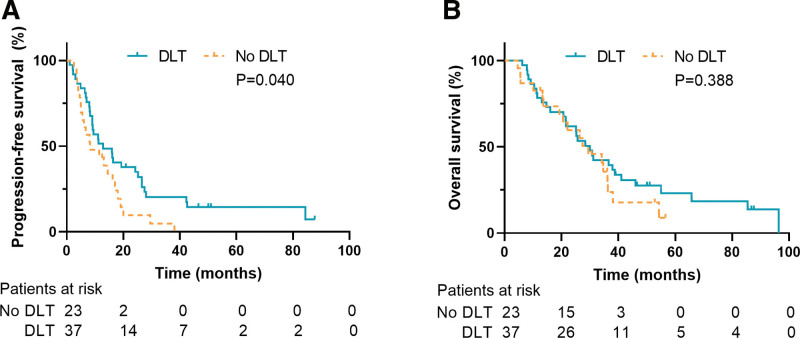
The subgroup survival analysis of patients without specific TKI therapy is shown in Figure 3. In this subgroup, the median and 5-year PFS of the no DLT group were 8.3 months and 0%. The median and 5-year OS were 29.4 months and 8.9%. For DLT group, the median and 5-year PFS were 12.9 months and 14.6%. The median and 5-year OS were 30.0 months and 23.0%. The log-rank test indicated that for the subgroup without specific TKI therapy, the PFS of patients receiving DLT was significantly superior (P = .040), but there was no significant difference in OS (P = .388). The Z test indicated that the 5-year PFS of patients receiving DLT was significantly improved (14.6% vs 0%, P = .021). Although the 5-year OS tended to be improved by DLT, the P value was not significant (23.0% vs 8.9%, P = .171).
Figure 3.
The subgroup survival analysis of patients without specific tyrosine kinase inhibitors therapy. (A) Progression-free survival. (B) Overall survival. DLT = definitive local therapy.
Among all 81 patients, 6 patients (7.4%) achieved PFS of at least 5 years. The individual characteristics are shown in Table 2. As of the last follow-up, 3 of the 6 patients had not progressed, and 5 were still alive. Among the patients with specific TKI therapy, 4 patients reached 5-year PFS, 2 of whom did not receive any local treatment and had progressed. Of the remaining 2 patients, 1 received SBRT for pulmonary metastases, and 1 received palliative radiotherapy for osseous metastasis (45 Gy/18 F). Except for the 5th patient in the table, only 1 patient with a sensitive mutation received both DLT and TKI. His oligorecurrent lesion was a pleural nodule and reached 38.5 months PFS without progression.
Table 2.
Characteristics for patients with ≥5 yrs PFS.
| No. | Site of recurence | DLT | Specific TKI therapy | PFS-time (mo) | Progression | Decease |
|---|---|---|---|---|---|---|
| 1 | Liver | Surgery | No | 87.7 | No | No |
| 2 | Bone | No# | Icotinib | 86.5 | No | No |
| 3 | Adrenal gland | RT 50Gy/25F | No | 84.4 | Yes | Yes |
| 4 | Lung | No | Gefitinib | 71.7 | Yes | No |
| 5 | Lung | SBRT 50Gy/10F | Icotinib | 65.8 | No | No |
| 6 | Adrenal gland | No | Crizotinib | 60.4 | Yes | No |
ALK = anaplastic lymphoma kinase, DLT = definitive local therapy, EGFR = epidermal growth factor receptor, PFS = progression-free survival, SBRT = stereotactic body radiotherapy, TKI = tyrosine kinase inhibitor.
#Note: No.2 patient receive palliative radiotherapy for 45Gy/18F.
3.5. Univariate and multivariate analysis
The univariate Cox regression results (Table 3) showed that for 81 patients, factors significantly related to PFS included sex (P = .020), initial TNM stage (P = .050), smoking history (P < .001), pathology type (P = .003) and specific TKI therapy (P = .003). Factors significantly related to OS included sex (P = .048), initial TNM stage (P = .029), smoking history (P = .007), pathological type (P < .001) and specific TKI therapy (P = .004).
Table 3.
Univariable cox regression.
| Characteristics | PFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| DLT | 0.899 (0.560–1.445) | .661 | 1.158 (0.688–1.948) | .581 |
| Male | 1.984 (1.112–3.539) | .020* | 1.947 (1.005–3.773) | .048* |
| Age < 60 | 0.777 (0.458–1.321) | .352 | 0.782 (0.439–1.391) | .402 |
| More advanced initial TNM stage | 1.323 (1.000–1.750) | .050* | 1.405 (1.035–1.908) | .029* |
| RI < 2 yrs | 1.697 (0.864–3.333) | .125 | 2.158 (0.924–5.040) | .075 |
| ECOG PS 0-1 | 0.826 (0.297–2.293) | .714 | 1.220 (0.380–3.916) | .738 |
| Solitary lesion | 1.426 (0.811–2.505) | .217 | 1.841 (0.928–3.651) | .081 |
| Smoking history | 2.570 (1.525–4.332) | <.001* | 2.203 (1.236–3.928) | .007* |
| ADC | 0.480 (0.295–0.779) | .003* | 0.359 (0.209–0.619) | <.001* |
| Specific TKI therapy | 0.413 (0.231–0.740) | .003* | 0.367 (0.184–0.731) | .004* |
ADC = adenocarcinoma, DLT = definitive local therapy, ECOG PS = Eastern Cooperative Oncology Group Performance status, HR = hazard ratio, OS = overall survival, PFS = progression-free survival, RI = recurrent interval, TKI = tyrosine kinase inhibitor.
P < .05.
A multivariate analysis was carried out based on the results of univariate analysis (Table 4). The results showed that DLT (HR, 0.469; 95% CI, 0.262–0.837; P = .010) and specific TKI therapy (HR, 0.442; 95% CI, 0.206–0.950; P = .036) were favorable prognostic factors significantly related to PFS. No prognostic factors were significantly related to OS.
Table 4.
Multivariable cox regression.
| Characteristics | PFS | OS | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| DLT | 0.469 (0.262–0.837) | .010* | 0.806 (0.443–1.466) | .480 |
| Male | 1.013 (0.475–2.161) | .973 | 1.196 (0.504–2.841) | .685 |
| More advanced Initial TNM stage | 1.227 (0.917–1.640) | .168 | 1.320 (0.953–1.828) | .095 |
| Smoking history | 1.227 (0.917–1.640) | .059 | 1.320 (0.953–1.828) | .457 |
| ADC | 0.613 (0.342–1.097) | .099 | 0.537 (0.279–1.034) | .063 |
| Specific TKI therapy | 0.442 (0.206–0.950) | .036* | 0.573 (0.232–1.412) | .226 |
ADC = adenocarcinoma, DLT = definitive local therapy, HR = hazard ratio, OS = overall survival, PFS = progression-free survival, TKI = tyrosine kinase inhibitor.
P < .05.
4. Discussion
Regarding the cause of oligometastasis, Hellman believed that in the early stage of the disease, malignant tumor cells had limited invasiveness, which led to directional metastasis to certain organs due to the physiological characteristics of tumor cells. The process is known as the “seed and soil” hypothesis.[8] In addition, the oligometastatic state in some patients was caused by systemic therapy. Some metastases become residual lesions that have not been eliminated by drugs due to their large tumor burden or drug resistance, resulting in oligometastasis.[2] For oligometastatic NSCLC patients, some retrospective studies have demonstrated that effective treatment of the primary tumor can improve prognosis.[9–11] Therefore, there is a view that oligometastatic patients with well-controlled primary lesions can be individually defined as having oligorecurrence and their treatment should try to achieve the goal of curing the condition.[5] For oligometastatic NSCLC patients, although the brain is one of the most common metastatic sites,[9] local therapy for brain metastases has been considered the standard treatment.[12] In addition, the blood–brain barrier and its special microenvironment promote distinct treatment responses in intracranial and extracranial metastatic lesions.[13,14] Therefore, we believe there is no comparison between intracranial and extracranial oligometastases. In terms of the extent of metastasis, a real-world study of 11,094 patients showed that the overall survival of NSCLC patients with single-organ metastases was better than that of those with multiorgan metastases.[15] Moreover, patients with single-organ metastases have a greater chance of receiving radical treatment. Therefore, we infer that extracranial single-organ oligorecurrent NSCLC patients have a greater potential to be cured. As a result, the present study assessed this group of patients to explore their prognostic characteristics and the role of DLT.
At present, there has been some prospective evidence for the application of DLT in the treatment of oligometastatic NSCLC patients. A single-center phase II study by Iyengar et al enrolled 29 patients with oligometastatic NSCLC who responded to induction chemotherapy and were randomized to receive stereotactic ablative radiotherapy (SABR) plus maintenance chemotherapy or maintenance chemotherapy alone. As a result, the PFS of the SABR group was significantly superior (P = .01).[16] Similarly, a multi-institutional, phase II, randomized study conducted by Gomez et al included a total of 49 patients. The patients were all oligometastatic NSCLC patients without progression at 3 or more months after front-line systemic therapy. Patients were randomly assigned to the local consolidative therapy group or to the maintenance therapy/observation group. The median PFS were 14.2 months and 4.4 months, respectively (P = .022), and the median OS were 41.2 months and 18.9 months (P = .017).[17] For oligorecurrent NSCLC, the current evidence is mainly retrospective.[18–21] An earlier study analyzed 28 patients with NSCLC who underwent resection of a single recurrent lesion after radical resection and found that the 5-year survival rate was 32%.[18] Yano et al conducted a prospective observational study on postoperative oligorecurrent NSCLC patients. The results showed that the median PFS of 11 patients who received local therapy was 20 months, and 5 of them achieved PFSs of more than 2 years.[12] Two retrospective studies by Hishida et al and Matsuguma et al also included NSCLC patients with oligorecurrence after surgery and concluded that DLT improved postprogression OS and PFS, but both included patients with intracranial oligorecurrence.[20,21] Moreover, most of the previous studies of oligorecurrent NSCLC only included patients who underwent radical surgery. In fact, SBRT and radical radiotherapy are also standard curative methods for NSCLC, and patients receiving SBRT and radical radiotherapy may also face oligorecurrence.
This study included 81 NSCLC patients with extracranial single-organ oligorecurrence using preset inclusion and exclusion criteria. Patients had previously received curative treatment, including SBRT, surgery and radiotherapy. Thirty-nine patients received DLT after oligorecurence. The results of the baseline comparison showed that the 2 groups of patients were unevenly distributed in terms of specific TKI therapy administered (P < .001), indicating that clinicians are more inclined to give DLT to patients who cannot choose specific TKI therapy. Survival analysis of all patients showed that DLT did not improve PFS (P = .664) or OS (P = .581), which may be due to the uneven distribution of specific TKI therapy. Further analysis confirmed our conjecture. In multivariate Cox regression, DLT (HR, 0.469; P = .010) and specific TKI therapy (HR, 0.442; P = .036) were the only 2 favorable prognostic factors significantly associated with PFS. This confirmed the value of DLT in improving PFS in extracranial single-organ oligorecurrent NSCLC.
In addition to local therapy, the role of systemic therapy in oligometastatic NSCLC cannot be ignored. With the development of targeted therapy and immunotherapy, there are more treatment options for advanced NSCLC patients. How to combine drugs and DLTs to obtain a more ideal long-term prognosis deserves attention. The SINDAS study included patients with EGFR-mutated extracranial oligometastatic NSCLC. Patients received stereotactic radiotherapy for both primary and metastatic lesions combined with targeted therapy. Interim analysis showed that whole-lesion stereotactic radiotherapy significantly improves PFS and OS.[22] In contrast to the design of SINDAS, the target population of our study was oligorecurrent NSCLC. Unfortunately, only 2 patients received both DLT and specific TKI therapy. Nonetheless, we found that these 2 patients achieved ideal PFS of 65.8 months and 38.5 months without progression. Moreover, among the patients who obtained PFS for more than 5 years, 3 patients received only TKI therapy without DLT, 2 of these 3 patients had progressed, and the only patient who did not progress received 45 Gy/18F palliative radiotherapy for osseous metastasis. Such results suggested to some extent that for extracranial oligorecurrent NSCLC patients, TKI therapy alone may achieve long-term PFS, but it is still difficult to achieve the goal of cure. TKI combined with local therapy may be a more promising choice. It should be noted that the screening failure rate of SINDAS is as high as 78%.[23] The feasibility of SBRT for primary lesions may be one of the reasons. However, in patients with oligorecurrence, the primary tumor was well controlled, and whether the SINDAS pattern can achieve better results needs to be confirmed by further studies.
In a subgroup analysis of patients not receiving specific TKI therapy, DLT significantly improved PFS (P = .040). The 5-year PFS rates in the 2 groups were 14.6% and 0%, respectively (P = .021). In terms of the 5-year OS rate, although the difference was not significant (P = .171), 23.0% in the DLT group showed a nearly 3-fold improvement trend compared to 8.9% in the non-DLT group. This result suggests that for the nonsensitive mutation subgroup, DLT is expected to improve long-term prognostic outcome. With the rise of immunotherapy, options for patients with non-EGFR/ALK mutations will no longer be limited to chemotherapy.[24] The combination of immunotherapy and local therapy, especially in combination with radiotherapy, has attracted much attention. This treatment combination based on the immune-stimulating effect of radiation.[25] The PEMBRO-RT study and the MDACC study included patients with metastatic NSCLC who received local radiotherapy with or without pembrolizumab. The results of the pooled analysis showed that the combined treatment group achieved an abscopal response rate of 41.7% and an abscopal disease control rate of 65.3%. The median PFS and OS were 9.0 months and 19.2 months for the experimental group, respectively, which were significantly better than those of the control group.[26] Based on such results, it is necessary to pay attention to whether the application of immunotherapy combined with radiotherapy in patients with oligorecurrence can further improve the prognosis.
The limitations of our study mainly stem from its retrospective nature. First, the number of enrolled patients was limited. Therefore, the conclusions of the study must be interpreted carefully and should be further verified by prospective studies. Second, the correlation between DLT and OS has not been confirmed. The reason may be that, in retrospective studies, too many factors, including the patient’s later-line treatment, long-term treatment toxicity, etc, will have an impact on OS, and the specific information on these factors for some patients was unknown, which may interfere with the results of OS.
5. Conclusion
For extracranial single-organ oligorecurrent NSCLC, PFS was significantly superior in patients receiving DLT. Among them, for the subgroup of patients who did not receive specific TKI therapy, DLT is expected to improve long-term prognostic outcomes. DLT combined with TKI may be an ideal treatment option to achieve clinical cure. It is necessary to further explore the strategy and value of DLT combined with different systemic therapies.
Author contributions
Conceptualization: Jia-Nan Jin, Peng Yue, Zheng-Bo Song, Ming Chen.
Data curation: Jia-Nan Jin, Peng Yue, Yue Hao, Shi-Yan Wu, Bai-Qiang Dong, Qing Wu.
Formal analysis: Jia-Nan Jin, Peng Yue, Zheng-Bo Song.
Funding acquisition: Zheng-Bo Song.
Investigation: Jia-Nan Jin, Peng Yue.
Methodology: Jia-Nan Jin, Peng Yue.
Project administration: Zheng-Bo Song, Ming Chen.
Resources: Ming Chen.
Software: Jia-Nan Jin.
Supervision: Zheng-Bo Song, Ming Chen.
Validation: Peng Yue, Zheng-Bo Song, Ming Chen.
Visualization: Zheng-Bo Song, Ming Chen.
Writing – original draft: Jia-Nan Jin, Peng Yue, Zheng-Bo Song, Ming Chen.
Writing – review & editing: Jia-Nan Jin, Peng Yue, Zheng-Bo Song, Ming Chen.
Abbreviations:
- ALK =
- anaplastic lymphoma kinase
- CT =
- computed tomography
- DLT =
- definitive local therapy
- EGFR =
- epidermal growth factor receptor
- MPLC =
- metachronous second primary lung cancer
- NGS =
- next generation sequencing
- NSCLC =
- non-small-cell lung cancer
- OS =
- overall survival
- PFS =
- progression-free survival
- SBRT =
- stereotactic body radiotherapy
- TKIs =
- tyrosine kinase inhibitors
The authors have no conflicts of interest to disclose.
This work was funded by the Foundation of CSCO-Shiyao (Y-SY201901-0068, to Zheng-Bo Song).
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
JJ and PY contributed equally to this work.
How to cite this article: Jin J-N, Yue P, Hao Y, Wu S-Y, Dong B-Q, Wu Q, Song Z-B, Chen M. Definitive local therapy for extracranial single-organ oligorecurrent non-small-cell lung cancer: A single institutional retrospective study. Medicine 2022;101:46(e31918).
Contributor Information
Jia-Nan Jin, Email: hzjjn0930@126.com.
Peng Yue, Email: yuepeng1995425@qq.com.
Yue Hao, Email: deartreasurealicia@163.com.
Shi-Yan Wu, Email: wuqing202020@163.com.
Bai-Qiang Dong, Email: dongbai111@163.com.
Qing Wu, Email: wuqing202020@163.com.
Ming Chen, Email: chenming@sysucc.org.cn.
References
- [1].Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. [DOI] [PubMed] [Google Scholar]
- [2].Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- [3].Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. [DOI] [PubMed] [Google Scholar]
- [4].Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Niibe Y, Chang JY. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm Med. 2012;2012:261096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shen KR, Meyers BF, Larner JM, et al. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:290S290S290s–305S. [DOI] [PubMed] [Google Scholar]
- [8].Price JE, Aukerman SL, Fidler IJ. Evidence that the process of murine melanoma metastasis is sequential and selective and contains stochastic elements. Cancer Res. 1986;46:5172–8. [PubMed] [Google Scholar]
- [9].Lopez Guerra JL, Gomez D, Zhuang Y, et al. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int J Radiat Oncol Biol Phys. 2012;84:e61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mordant P, Arame A, De Dominicis F, et al. Which metastasis management allows long-term survival of synchronous solitary M1b non-small cell lung cancer? Eur J Cardiothorac Surg. 2012;41:617–22. [DOI] [PubMed] [Google Scholar]
- [11].Petrelli F, Ghidini A, Cabiddu M, et al. Addition of radiotherapy to the primary tumour in oligometastatic NSCLC: a systematic review and meta-analysis. Lung Cancer. 2018;126:194–200. [DOI] [PubMed] [Google Scholar]
- [12].Yano T, Okamoto T, Haro A, et al. Local treatment of oligometastatic recurrence in patients with resected non-small cell lung cancer. Lung Cancer. 2013;82:431–5. [DOI] [PubMed] [Google Scholar]
- [13].Bulbul A, Forde PM, Murtuza A, et al. Systemic treatment options for brain metastases from non-small-cell lung cancer. Oncology (Williston Park). 2018;32:156–63. [PubMed] [Google Scholar]
- [14].Kudo Y, Haymaker C, Zhang J, et al. Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small-cell lung cancer. Ann Oncol. 2019;30:1521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hendriks LE, Derks JL, Postmus PE, et al. Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: results from a population-based study. Eur J Cancer. 2015;51:2534–44. [DOI] [PubMed] [Google Scholar]
- [16].Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hishida T, Nagai K, Yoshida J, et al. Is surgical resection indicated for a solitary non-small cell lung cancer recurrence? J Thorac Cardiovasc Surg. 2006;131:838–42. [DOI] [PubMed] [Google Scholar]
- [19].Yano T, Haro A, Yoshida T, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol. 2010;102:852–5. [DOI] [PubMed] [Google Scholar]
- [20].Hishida T, Yoshida J, Aokage K, et al. Postoperative oligo-recurrence of non-small-cell lung cancer: clinical features and survival. Eur J Cardiothorac Surg. 2016;49:847–53. [DOI] [PubMed] [Google Scholar]
- [21].Matsuguma H, Nakahara R, Wakamatsu I, et al. Definitive local therapy for oligo-recurrence in patients with completely resected non-small cell lung cancer. Am J Clin Oncol. 2020;43:210–7. [DOI] [PubMed] [Google Scholar]
- [22].Wang X, Zeng M. First-line tyrosine kinase inhibitor with or without aggressive upfront local radiation therapy in patients with EGFRm oligometastatic non-small cell lung cancer: interim results of a randomized phase III, open-label clinical trial (SINDAS) (NCT02893332). J Clin Oncol. 2020;38:9508–9508. [Google Scholar]
- [23].Mielgo-Rubio X, Garde-Noguera J, Juan O, et al. Stereotactic body radiation therapy: a good dance partner of oligometastatic non-small cell lung cancer to the sound of SINDAS study. World J Clin Oncol. 2020;11:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. [DOI] [PubMed] [Google Scholar]
- [25].Daly ME, Monjazeb AM, Kelly K. Clinical trials integrating immunotherapy and radiation for non-small-cell lung cancer. J Thorac Oncol. 2015;10:1685–93. [DOI] [PubMed] [Google Scholar]
- [26].Theelen W, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9:467–75. [DOI] [PubMed] [Google Scholar]