Abstract
Sleep disturbances are associated with cold hypersensitivity (CH) and characterized by excessive cold sensation in specific body parts and cold thermal discomfort. This study investigated the effects of short-term sleep restriction followed by a recovery phase on subjective health status, inflammation, and lipid metabolism in different types of CH. A total of 118 healthy adults aged 35 to 44 years without sleep disturbances were enrolled. Participants underwent 4-hour sleep restrictions per day for 3 days at a hospital and then returned to their daily lives for 4 days of rest. CH was assessed using a structured questionnaire with eight characteristic symptoms. A questionnaire and blood tests were administered baseline, after sleep restriction, and follow-up to assess cortisol, lipid profiles, and self-reported stress and quality of life (QOL). Participants were divided into CH (44.1%) and non-CH (55.9%) groups. The CH group showed increased stress, impaired QOL, and decreased low-density lipoprotein-cholesterol (LDL-C) levels compared to the non-CH group after sleep restriction. The variance for QOL (effect size = 0.07), subjective stress (effect size = 0.053), and LDL-C (effect size = 0.029) among time points depended on the group. Short-term sleep restriction was associated with deterioration of subjective health and reduced lipid metabolism; such changes were more evident in the CH group. Our findings suggest the need to consider an individual’s CH status to assess the clinical risk associated with insufficient sleep.
Keywords: cold hypersensitivity, cortisol, lipids, quality of life, sleep restriction, stress
1. Introduction
Inadequate sleep has been associated with an increased risk of cardiovascular disease,[1,2] and has an adverse impact on quality of life (QOL), metabolism, and the immune system.[3] Furthermore, sleep is associated with the body’s thermoregulation ability,[4] which is closely associated with distal and proximal skin temperature, as well as core body temperature.[5] Twenty-four-hour sleep deprivation disrupts the coordination of thermoregulatory skin temperature fluctuations in the hands and feet, leading to increased heat loss in the feet and reduced heat loss in the hands.[6]
Individuals with cold extremities, such as cold hypersensitivity or thermal discomfort in the hands and feet but otherwise healthy, experience sleep disturbances such as prolonged sleep onset, delayed phases of circadian rhythm, and diminished sleep quality.[7–10] People with intense cold extremity symptoms have a two-fold higher relative risk for difficulty initiating sleep[8] and a 1.46 increase in odds ratio of poor sleep quality.[11] The prolonged sleep-onset latency of healthy individuals with cold feet was believed to be related to damaged distal vasodilatory capacity.[9] Moreover, in a 40-h sleep deprivation experiment, women with cold extremities and difficulties initiating sleep showed approximately 1 h of phase delay in the circadian rhythm and significantly longer sleep-onset latency compared to a control group without these features.[10] Although both groups had a similar habitual bedtime, this supported the hypothesis that the circadian physiology of individuals with cold extremities and difficulty initiating sleep failed to adequately prepare their bodies for sleep initiation.[10]
Even at the same environmental temperature, humans show varying levels of temperature sensations, and Traditional East Asian Medicine (TEAM) defines this as a cold pattern or cold hypersensitivity (CH).[12] CH, one of the pattern identifications, is an essential component of the diagnostic process in TEAM, commonly manifested by aversion to or fear of cold, preference for warm temperatures, and cold-type symptoms and signs.[12] CH has been associated with high norepinephrine levels and low changes in glucose metabolism[13] and a low risk for metabolic syndrome due to the activation of adiponectin.[14] Conversely, CH has also been associated with deterioration in the quality of life.[7] Despite some attempts to explore the physiological factors of CH and the association between CH and general health, to the best of our knowledge, experimental studies investigating the physiological and psychological changes in humans because of sleep status, which is strongly associated with CH, are lacking.
Our hypothesis is that the physiological and psychological changes caused by insufficient sleep will differ according to CH. This study investigated the changes in biomarkers, such as cortisol and lipid profiles, and self-reported health status resulting from sleep restriction (SR) in relation to CH in healthy adults.
2. Materials and methods
2.1. Study design
This was an uncontrolled, before-and-after study with 3 days of 4-hour SR as intervention at two Korean medicine hospitals from December 2014 to March 2015. The protocol of this study has been previously described in detail.[15] Briefly, three assessments were obtained: baseline (before SR, day 1), after SR with short sleep duration at hospitals (day 3), and at the follow-up (day 8) after the recovery phase at the individual’s home. During the SR phase of 3 days and 2 nights, all participants received the same 4 hours of sleep per day allowed only between 1 am and 5 am at hospitals, and were systematically managed with standard hospital meals, caffeine, snack, and daytime activities including naps. During the recovery phase of 4 days and 3 nights, participants were rested and slept at will with their normal amount of sleep at their own home, and their daily sleep duration was recorded using a sleep diary. This study was approved by the Institutional Review Board of each participating institution (IRB No. KOMCIRB-140923-HR-007, KHNMCOH 2014-09-010), and written informed consent was obtained from all participants. The protocol of this clinical trial was registered at the Clinical Research Information Service (KCT0001503).
2.2. Participants
Individuals between 35 and 44 years with a normal body mass index (BMI) (between 18.5 and 25 kg/m2) and normal sleep status (an average sleeping time between 7 and 8 hours, and a Pittsburgh Sleep Quality Index score ≤ 5) were enrolled. Individuals were excluded if they had undergone treatment for any internal, neurological, or psychological disorder within 6 months prior to the study or presented evidence of sleep disorders or cardiovascular, cerebrovascular, or respiratory disorders through an interview with a physician and from their medical history. Individuals who experienced severe fatigue or pain, were currently smoking, pregnant, breastfeeding, menstruating, or participating in other clinical trials were also excluded. A total of 130 participants completed the study. Of these, 118 participants who completed the entire study procedure were included in the final analysis (12 failed to apply the sleep restriction per protocol).
2.3. Data collection and measures
2.3.1. Definition of cold hypersensitivity.
CH was evaluated using the Cold-Heat Pattern Identification questionnaire[16] with eight characteristic symptoms at baseline: aversion to chills or cold, such as a preference for warmth or warm temperature, tendency to feel chilly in the stomach, tendency to have cold hands and feet, feeling cold in the body, having a pale complexion, tendency to drink warm water, and having clear or transparent urine. The original questionnaire is acceptably reliable (Cronbach’s alpha = 0.79, correlation coefficient range = 0.79 via test-retest)[17] and valid (87.1% agreement, compared to two professional’s examination).[16] Questions were evaluated on a four-point scale as follows: 4 = strongly agree, 3 = agree, 2 = disagree, and 1 = strongly disagree. The CH score was calculated as the sum of the eight items, which ranged from 8 to 32, with a higher score indicating a higher risk of cold hypersensitivity. Participants were categorized into the CH group (CH score > 20) and the non-CH group (CH score ≤ 20) based on median value of the CH score. The questionnaire used in this study had the same items as the original tool, but a different scale (five-point scale in the original tool); therefore, the cutoff criterion of the original tool could not be applied. The Cronbach’s alpha for total CH scale was 0.683 in this study.
2.3.2. Blood sampling of cortisol and lipid metabolic biomarkers.
The cortisol and lipid profiles, such as total cholesterol, high-density lipoprotein-cholesterol, and low-density lipoprotein-cholesterol (LDL-C), were measured by blood tests at baseline, after SR, and follow-up. Blood tests were performed according to the standard protocol in the early morning after ≥ 8 hours of fasting. Cortisol was measured using the ADVIA Centaur XP (Siemens Healthcare Diagnostics Inc., NY), and the remaining parameters were measured using the ADVIA1800 instrument (Siemens Healthcare Diagnostics Inc.).
2.3.3. Self-reported health status.
Subjective health status, including stress and QOL, was assessed using a self-administered questionnaire at baseline, after SR, and follow-up. The psychosocial well-being index-short form (PWI-SF) measures stress levels and consists of 18 items.[18] Each item was evaluated on a four-point response scale from 0 to 3, with the total score ranging from 0 to 54. Higher scores indicate a worse level of stress. The Cronbach’s alpha for the PWI-SF scale was 0.92 in this study. The EQ-5D measures general health-related quality of life of five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) with three levels.[19] The EQ-5D provides an estimate of the health summary score by applying a formula that assigns specific values to each level in each dimension. In this study, EQ-5D scores were calculated using the time trade-off valuation set that pertains to the Korean population.[20] The total score ranges from 0 to 1, with a lower score indicating a poorer health status.
2.4. Statistical analysis
General characteristics are described in terms of frequency, mean, and standard deviation using Fisher’s exact test or independent t tests between the CH and non-CH groups at baseline. The Shapiro–Wilk test was applied to analyze normality to identify the similarity of both groups at baseline. The Pearson correlation coefficient was used to examine a relationship between CH score and the change value of each variable, the difference between baseline and after SR, and that between baseline and follow-up. An analysis of covariance was performed to assess the intervention effects at sleep restriction and follow-up compared to the values at baseline between the two groups, after adjusting for age, sex, and baseline values of each outcome variable. A two-way repeated-measures analysis of variance was used to explore the interaction effect between time (baseline, after SR, and follow-up) and group (CH and non-CH), providing the effect size as partial eta square values. The assumption of sphericity was tested using Mauchly’s test, and the Greenhouse–Geisser approach were used for correction when the model did not adequately fit. A partial eta squared value of 0.01, 0.06, and 0.14 corresponds to a small, medium, and large effect size, respectively.[21] Missing data were replaced with the mean value of the corresponding day of visit (one variable for the CH questionnaire and two variables for PWI-SF). All statistical analyses were performed using SPSS software version 24.0 (IBM Corp., NY) and statistical significance was set at a P < .05.
3. Results
3.1. General characteristics of participants
Of the 118 participants, 52 were in the CH group (44.1%), and 66 in the non-CH group (55.9%). The two groups showed significant differences in sex, BMI, QOL and cortisol level at baseline. The CH group had a greater proportion of female participants (male 23.1% vs female 76.9%), and the non-CH group a higher proportion of male participants (male 66.7% vs female 28.8%). BMI and cortisol levels were significantly higher in the non-CH group than in the CH group, whereas QOL was lower in the CH group than in the non-CH group (Table 1 and Supplemental Digital Content 1, http://links.lww.com/MD/H985).
Table 1.
Baseline characteristics of study participants.
| Variables | CH group | Non-CH group | P value* |
|---|---|---|---|
| Sex (M/F) | 52 (12/40) | 66 (47/19) | <.001 |
| Age (yr) | 39.5 ± 3.3 | 39.2 ± 3.0 | .608 |
| BMI (kg/m2) | 21.4 ± 2.0 | 22.8 ± 1.8 | <.001 |
| Sleep quality | 4.0 ± 2.4 | 4.2 ± 1.4 | .676 |
Data are shown as numbers for sex and as mean ± standard deviation (SD) for continuous variables.
Sleep quality were measured using the Pittsburgh Sleep Quality Index.
BMI = body mass index, CH = cold hypersensitivity, F = female, M = male.
P values for sex are based on Fisher’s exact test; all other P values for continuous variables are based on independent t tests between the CH and non-CH groups.
3.2. Correlation between CH score and changes in each variable
The CH score was weakly correlated with cortisol (r = 0.2, P = .03) and LDL-C (r = −0.236, P = .01), measured by changes of each variable between after SR and baseline. After recovery, the CH score was positively correlated with change in QOL (r = 0.386, P < .001), which were changes of each variable between follow-up and baseline (Table 2).
Table 2.
Correlation between CH score and changes in each variable during sleep restriction and follow-up.
| Change of variables | CH score | |
|---|---|---|
| After SR to baseline (r) | Follow-up to baseline (r) | |
| Δ Stress | 0.174 | −0.050 |
| Δ QOL | −0.006 | 0.386** |
| Δ Cortisol | 0.200* | 0.081 |
| Δ TC | −0.155 | 0.065 |
| Δ HDL-C | −0.112 | 0.040 |
| Δ LDL-C | −0.236* | 0.067 |
Δ (delta) of changes in each variable during sleep restriction and follow-up, respectively.
All correlations represent associations between CH score and changes in each variables.
CH = cold hypersensitivity, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, QOL = quality of life, SR = sleep restriction, TC = total cholesterol.
P < .05,
P < .001.
3.3. Effects of sleep restriction and follow-up on variables in the CH and non-CH groups
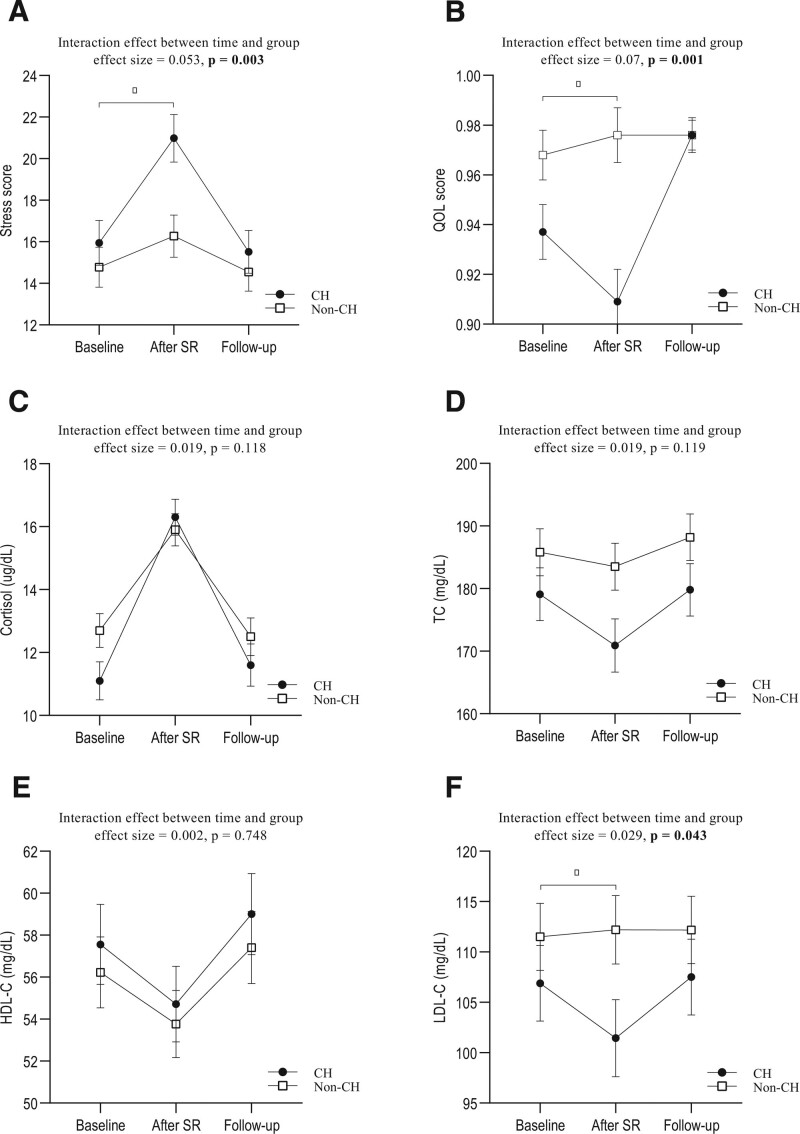
The analysis of covariance analysis showed that SR led to changes in stress, QOL, and LDL-C, showing significant differences between the CH and non-CH groups. In the CH group, stress was increased, whereas QOL and LDL-C reduced compared to those of the non-CH group. However, after the recovery phase, the values at follow-up were similar to the baseline values. This indicated that variables recovered to their pre-SR states after the recovery phase in both groups (Fig. 1A–F and Table S1, Supplemental Digital Content, http://links.lww.com/MD/H985).
Figure 1.
Interaction effect of time and group for variables. (A) stress, (B) QOL, (C) cortisol, (D) TC, (E) HDL-C, and (F) LDL-C. Data are shown as estimated mean and standard error. *P < .05 after sleep restriction, using ANCOVA analysis adjusted for sex, age, and baseline values of each outcome variable. ANCOVA = an analysis of covariance, CH = cold hypersensitivity, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, QOL = quality of life, SR = sleep restriction, TC = total cholesterol.
A significant interaction effect between time (baseline, after SR, and follow-up) and group (CH and non-CH group) was observed for stress, QOL, and LDL-C and, indicating that these variables showed different changes between groups over time. The effect sizes for the interactions were considered moderate in QOL (effect size = 0.07 in Fig. 1B) and small in stress (effect size = 0.053 in Fig. 1A) and LDL-C (effect size = 0.029 in Fig. 1F).
4. Discussion
This study observed changes in subjective health status, cortisol, and lipid levels after two nights of SR and four consecutive days of recovery in healthy early middle-aged adults, particularly with regard to CH, which is associated with sleep disturbances.
We confirmed that less-than-optimal sleep duration leads to worsened stress and QOL and reduced LDL-C levels. These changes were significant in the CH group compared to the non-CH group, when examined according to the classification of CH score. The changes brought upon by SR returned to normal levels after resting for a period longer than that of the sleep duration, regardless of the CH status. Furthermore, the variance observed for stress, QOL, and LDL-C among time points was dependent on the group, suggesting that these variables exhibited a significant small-to-moderate interaction effect between time and group. The significance of our findings is that insufficient sleep duration could induce negative effects on subjective health level and altered lipids metabolism, even in healthy adults, and that the changes differed between people with CH, a condition characterized by the individual’s perceived thermal sensation and preferred temperature.
A study in Korea showed that cold extremities had negative effects on physical and mental QOL.[7] In related work, another study revealed that young women with cold extremities showed reduced sleep quality and impaired QOL with fatigue and pain.[22] Previous findings are in line with ours, thus supporting them. Furthermore, in our experimental study of two groups with similar good sleep quality, we substantiated that inadequate sleep provokes a negative stress and QOL response in individuals with CH, which highlights that sleep disturbance is a major health problem in this population.
Changes in cortisol levels during SR were weakly correlated with CH score, suggesting that a CH state with a higher CH score was related to an increase in cortisol. In general, studies that experimented with 24- to 40-hour total sleep deprivation observed elevated cortisol levels.[23,24] Our findings showed that even short-term SR contributed to changes in cortisol levels, this result support previous findings and present additional evidence. Cortisol is the body’s response to a stressor, and it is released into the blood in response to stress. Sleep deprivation is considered a physiological stressor and metabolic challenge, and it is especially associated with increased cortisol concentration and stress parameters in the early evening and early morning hours.[23,24] Furthermore, genetic factors related to cold sensitivity were founded in the latest study, they regulated expression and function of cold-inducible RNA-binding-protein in inflammatory response.[25] Although we did observe that individuals close to CH status had small negative stress responses to inadequate sleep, there were no clearly significant differences in inflammatory markers between the CH and non-CH groups. Further studies will be needed considering CH-related epigenetic processes in sleep disturbances.
Changes of LDL-C during SR had a weak negative correlation with CH score. These results suggest that the decrease in LDL-C related to insufficient sleep duration was higher in the CH group than in the non-CH group. Although the mechanism of circadian regulation in lipid metabolism in humans is unknown,[26] several SR studies have shown controversial impact on changes in lipid metabolism. A study applying five nights of 4-hour SR in normal-weight adults found that SR did not affect the lipid profiles.[27] In contrast, a study with a similar design in postmenopausal women reported elevated total cholesterol and LDL-C levels.[28] Similar to our findings, an experimental study on sleep deprivation found that SR led to reduced LDL-C levels with no changes in high-density lipoprotein-cholesterol, suggesting that inadequate sleep altered lipoprotein metabolism, which was associated with reduced expression of genes encoding cholesterol receptors and increased inflammatory responses.[29] Low LDL-C levels after sleep deprivation are induced by acute phase inflammatory responses triggered by SR.[30] In this aspect, we observed that there was a marginal negative correlation between changes of LDL-C and change of cortisol during SR (correlation coefficient = −0.167, P = .071, result was not shown). Taken together, we can assume that altered LDL-C in the CH group is an acute response to the increase in stress, measured by cortisol and self-reported stress, caused by sleep loss. This assumption needs to be investigated further, including the health effects of changes in lipid metabolism caused by sleep problems.
The distribution of sex and BMI differed between the CH and non-CH groups in this study. In a study that defined the cold type based on peripheral and abdominal temperature sensations in the general population of Korea[13] and Switzerland,[8] the prevalence of cold extremities was approximately 3 to 4.5 times higher in women than in men. CH is associated with female sex, younger age, and low BMI[31]; in particular, BMI was negatively correlated with CH in women. Our results are in line with those of previous studies. Furthermore, our study defined CH close to that of the cold pattern defined by TEAM by including the preferred temperature and signs and symptoms of CH in addition to temperature sensation, which has often been used to define the cold type in previous studies.[7,13]
This study had several limitations. First, we could not consider differences in the participants’ usual dietary habits when interpreting SR and lipid metabolic parameters. However, we only enrolled nonsmoking participants with a normal BMI, and the research team strictly controlled diet and activity during the study period. Second, we did not examine participants’ normal sleep-wake patterns using polysomnography or actigraphy to investigate participants’ adherence to sleep restrictions during the experiment. However, all experiments were conducted in the same space of each hospital, and the investigator managed the lighting to ensure a similar light environment for all participants. Finally, we classified CH into two groups at the median value using a questionnaire, because it was not suitable for application of the cutoff criterion of original tool. Dichotomy led to several issues, such as missing information, underestimation of the extent of outcome variations between groups, and concealment of any non-linearity in the association between variable and outcome[32]; however, we considered that a median split was acceptable in the context of the study, particularly given the sample size. Considering the above weaknesses, subsequent studies should compare health outcome and metabolic changes while considering the participants’ usual lifestyle, use objective data to assess sleep states, and improve the CH classification process.
5. Conclusions
Sleep restriction causes psychological discomfort and alters lipid metabolism. These changes are more evident in subjective stress, QOL, and LDL-C indicator in people with CH, characterized by perceived hypersensitivity to cold temperatures and consequent discomfort. These results will provide additional information for evaluating the clinical risks posed by sleep disturbances and assessing the usual sleep patterns according to CH. Future studies should focus on the timing and changes of sleep patterns in accordance with features of CH and examine the bidirectional association between sleep disturbances and disease through long-term cohort studies.
Acknowledgments
This study was supported by grant from the Korea Institution of Oriental Medicine.
Author contributions
YB and SL designed the study, analyzed the data, and wrote the paper. KJ collected data, conducted quality control of the data, and wrote the manuscript. HK analyzed data. All authors have read and approved the final manuscript.
Conceptualization: Younghwa Baek, Siwoo Lee.
Data curation: Kyoungsik Jung.
Formal analysis: Younghwa Baek, Hoseok Kim.
Funding acquisition: Siwoo Lee.
Investigation: Younghwa Baek.
Methodology: Siwoo Lee.
Visualization: Younghwa Baek.
Writing – original draft: Younghwa Baek, Kyoungsik Jung, Hoseok Kim, Siwoo Lee.
Supplementary Material
Abbreviations:
- BMI =
- body mass index
- CH =
- cold hypersensitivity
- LDL-C =
- low-density lipoprotein cholesterol
- PWI-SF =
- psychosocial well-being index-short form
- QOL =
- quality of life
- SR =
- sleep restriction
- TEAM =
- Traditional East Asian Medicine
This study was supported by a “Development of Korean Medicine Original Technology for Preventive Treatment based on Integrative Big Data” grant from the Korea Institution of Oriental Medicine (no. KSN2023120).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Baek Y, Jung K, Kim H, Lee S. Partial sleep restriction-induced changes in stress, quality of life, and lipid metabolism in relation to cold hypersensitivity: A before-and-after intervention study. Medicine 2022;101:46(e31933).
Contributor Information
Younghwa Baek, Email: aori79@kiom.re.kr.
Kyoungsik Jung, Email: jksik@kiom.re.kr.
Hoseok Kim, Email: hodol9980@kiom.re.kr.
References
- [1].Cappuccio FP, Cooper D, D’Elia L, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. [DOI] [PubMed] [Google Scholar]
- [2].Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, et al. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Matricciani L, Bin YS, Lallukka T, et al. Past, present, and future: trends in sleep duration and implications for public health. Sleep Health. 2017;3:317–23. [DOI] [PubMed] [Google Scholar]
- [4].Kräuchi K, Cajochen C, Werth E, et al. Warm feet promote the rapid onset of sleep. Nature. 1999;401:36–7. [DOI] [PubMed] [Google Scholar]
- [5].Kräuchi K. The human sleep–wake cycle reconsidered from a thermoregulatory point of view. Physiol Behav. 2007;90:236–45. [DOI] [PubMed] [Google Scholar]
- [6].Romeijn N, Verweij IM, Koeleman A, et al. Cold hands, warm feet: sleep deprivation disrupts thermoregulation and its association with vigilance. Sleep. 2012;35:1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bae KH, Lee Y, Go HY, et al. The relationship between cold hypersensitivity in the hands and feet and health-related quality of life in Koreans: a nationwide population survey. Evid Based Complement Alternat Med. 2019;2019:6217036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kräuchi K, Gasio PF, Vollenweider S, et al. Cold extremities and difficulties initiating sleep: evidence of co-morbidity from a random sample of a Swiss urban population. J Sleep Res. 2008;17:420–6. [DOI] [PubMed] [Google Scholar]
- [9].Pache M, Kräuchi K, Cajochen C, et al. Cold feet and prolonged sleep-onset latency in vasospastic syndrome. Lancet. 2001;358:125–6. [DOI] [PubMed] [Google Scholar]
- [10].Vollenweider S, Wirz-Justice A, Flammer J, et al. Chronobiological characterization of women with primary vasospastic syndrome: body heat loss capacity in relation to sleep initiation and phase of entrainment. Am J Physiol Regul Integr Comp Physiol. 2008;294:R630–8. [DOI] [PubMed] [Google Scholar]
- [11].Seo B-N, Jeong K, Baek Y, et al. Study on the relationship between cold type and sleep quality in Koreans. J Physiol Pathol Korean Med. 2021;35:42–6. [Google Scholar]
- [12].World Health Organization. WHO international standard terminologies on traditional medicine in the western pacific region. 2007. Available at: https://apps.who.int/iris/handle/10665/206952 [access date January 3, 2022].
- [13].Pham DD, Lee J, Kim G, et al. Relationship of the cold-heat sensation of the limbs and abdomen with physiological biomarkers. Evid Based Complement Alternat Med. 2016;2016:2718051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park AY, Cha S. Effects of cold sensitivity in the extremities on circulating adiponectin levels and metabolic syndrome in women. BMC Complement Altern Med. 2017;17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baek Y, Jung K, Lee S. Effects of sleep restriction on subjective and physiological variables in middle-aged Korean adults: an intervention study. Sleep Med. 2020;70:60–5. [DOI] [PubMed] [Google Scholar]
- [16].Bae KH, Jang ES, Park G, et al. Development on the questionnaire of cold-heat pattern identification based on usual symptoms for health promotion – focused on agreement study. J Soc Prev Korean Med. 2016;20:17–26. [Google Scholar]
- [17].Yeo M, Park K, Bae K, et al. Development on the questionnaire of cold-heat pattern identification based on usual symptoms for health promotion – focused on reliability study. J Physiol Pathol Korean Med. 2016;30:112–23. [Google Scholar]
- [18].Chang S. Standardization of Collection and Measurement for Heath Data. Seoul, Korea: Kyechukmunhwasa, 2000:121–59. [Google Scholar]
- [19].Brooks R. EuroQol: the current state of play. Health Pol. 1996;37:53–72. [DOI] [PubMed] [Google Scholar]
- [20].Lee YK, Nam HS, Chuang LH, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health. 2009;12:1187–93. [DOI] [PubMed] [Google Scholar]
- [21].Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011;6:135–47. [Google Scholar]
- [22].Tsuboi S, Mine T, Tomioka Y, et al. Are cold extremities an issue in women’s health? Epidemiological evaluation of cold extremities among Japanese women. Int J Womens Health. 2019;11:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Joo EY, Yoon CW, Koo DL, et al. Adverse effects of 24 hours of sleep deprivation on cognition and stress hormones. J Clin Neurol. 2012;8:146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wright KP, Jr, Drake AL, Frey DJ, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim SY, Ban HJ, Lee S, et al. Regulation of CIRP by genetic factors of SP1 related to cold sensitivity. Front Immunol. 2022;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].O’Keeffe M, Roberts AL, Kelleman M, et al. No effects of short-term sleep restriction, in a controlled feeding setting, on lipid profiles in normal-weight adults. J Sleep Res. 2013;22:717–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kerkhofs M, Boudjeltia KZ, Stenuit P, et al. Sleep restriction increases blood neutrophils, total cholesterol and low density lipoprotein cholesterol in postmenopausal women: a preliminary study. Maturitas. 2007;56:212–5. [DOI] [PubMed] [Google Scholar]
- [29].Aho V, Ollila HM, Kronholm E, et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep. 2016;6:24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aho V, Ollila HM, Rantanen V, et al. Partial sleep restriction activates immune response-related gene expression pathways: experimental and epidemiological studies in humans. PLoS One. 2013;8:e77184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mozaffarieh M, Fontana Gasio P, Schötzau A, et al. Thermal discomfort with cold extremities in relation to age, gender, and body mass index in a random sample of a Swiss urban population. Popul Health Metr. 2010;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]