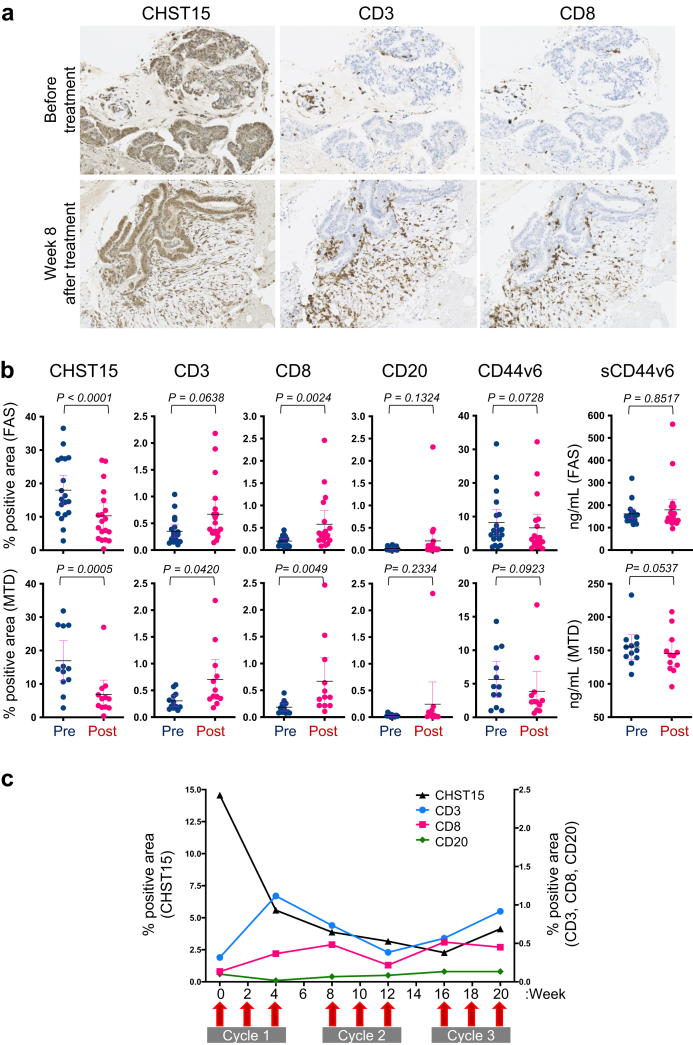
Fig. 4.
Changes in tumor microenvironmental CHST15 and lymphocytes.a. Immunostaining for CHST15 (brown, left panels), CD3 (brown, middle panels), and CD8 (brown, right panels) in tumor specimens obtained before (upper panels) and 8 weeks after the first injection (lower panels) of STNM01. b. Percentage positive CHST15+ (n = 19 in FAS, n = 12 in MTD), CD3+ (n = 19 in FAS, n = 12 in MTD), CD8+ (n = 18 in FAS, n = 12 in MTD), CD20+ (n = 19 in FAS, n = 12 in MTD), and CD44v6+ (n = 19 in FAS, n = 12 in MTD) areas, and serum sCD44v6 levels (n = 21 in FAS, n = 12 in MTD) at baseline (blue, W0 = week 0) and at the end of cycle 1 (magenta, W4 = week 4). Upper panels show FAS and lower panels show MTD population. Mean with 95%CI. P values determined by Wilcoxon matched-pairs signed rank test are shown. c. Kinetics of and positive areas for CHST15 (black line), CD3 (blue line), CD8 (magenta line), and CD20 (green line) in a patient receiving a maximum of 3 cycles of treatment with 10,000 nM STNM01.