Abstract
Mycorrhizae association is reported to enhance the survivability of the host plant under adverse environmental conditions. The present study aims to explore the mycorrhizal association in the roots of different ecotypes of a threatened medicinal plant, Clerodendrum indicum (L.) O. Kuntze (Verbenaceae), collected from W.B., India, which correlates the degree of root colonization to the nutritional status of the native soil. Ten ecotypes of C. indicum having diverse morphological variations were collected. The mycorrhizae were characterized by both morphological and molecular methods. The nutritional status of the native soils was estimated. The study revealed that all the ecotypes have an association with mycorrhizal forms like hyphae, arbuscules, and vesicles. The molecular analysis showed Glomus intraradices and Rhizophagus irregularis as the associated arbuscular mycorrhizal fungi (AMF). A significant variation in arbuscule and vesicle formation was found growing in the varied nutritional statuses concerning soil parameters. The arbuscule was found negatively correlated with pH, conductivity, and potassium and positively correlated with organic carbon, nitrogen, and phosphorus. The vesicle was found positively correlated with pH, organic carbon, and potassium and negatively correlated with conductivity, nitrogen, and phosphorus. The interaction between conductivity: nitrogen, conductivity: phosphorus, organic-carbon: nitrogen, and pH: conductivity was significant in influencing vesicle formation. However, none of the interactions between parameters was found significant in influencing arbuscule formation. Thus, the study concludes that G. intraradices and R. irregularis are the principle mycorrhizae forming the symbiotic association with the threatened medicinal plant, C. indicum. They form vesicles and arbuscules based on their soil nutritive factors. Therefore, a large-scale propagation through a selective AMF association would help in the conservation of this threatened species from extinction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-022-00805-2.
Keywords: Arbuscular mycorrhizal fungi, Glomus intraradices, Rhizophagus irregularis, Soil nutritional content
Introduction
Clerodendrum indicum (L.) O. Kuntze (Verbenaceae) (synonym: C. siphonanthus R. Br.; C. indicum f. semiserratum (Wall.) Moldenke; http://www.theplantlist.org/) is a highly valued woody perennial threatened medicinal plant native to India, commonly known as Bamanhati [1; https://indiabiodiversity.org/species/show/229224]. In India, it grows in Arunachal Pradesh, Assam, Bihar, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Manipur, Odisha, Sikkim, Tamil Nadu, Tripura, Uttar Pradesh, and West Bengal [2; https://indiabiodiversity.org/species/show/229224]. It is also found in East-Asian countries such as Southern Bangladesh, Cambodia, China, Indonesia, Laos, Malaysia, Myanmar, Nepal, Sri Lanka, Thailand, and Vietnam [3, 4].
The medicinal attributes of the plant are well known in different ethnic groups of India [2]. According to Ayurveda, it is an excellent anti-inflammatory agent and helps heal wounds [2, 5]. It improves the circulation of blood in the body [2]. The plant is used in fever, atrophy, consumption, cough, bronchitis, and blindness [2, 5]. North-East India traditionally uses aqueous leaf extract to alleviate diabetes, obesity, and hypertension symptoms [5–7]. The dried leaves are smoked like cigarettes to relieve asthma [5–7]. The juice of the tender parts of the plant is used as an external application for skin complaints [2]. The pounded root, combined with ginger, is considered helpful in treating asthma, coughs, and other pulmonary complaints and scrofulous affections [2]. A resin extracted from the plant is used to treat syphilitic rheumatism [2]. Extensive studies were carried out regarding the phytochemistry and medicinal uses of C. indicum [8–12]. The plant contains clerodendrone, cleroindicins, cleroindicin-α-hispidulin, clerodendrol, scutellarein, scutellarein-7-O-β-D-glucuronide-3, 4-dihydroxy phenyl ethanol, hispidulin 7-O-glucuronide, roseoside, eupafolin, lariciresinol, l9-O-beta-D-glucoside, 17-hydroxyteuvincenone G, and 17-hydroxyteuvincen-5(6)-enone G [8–12]. Different authors reported the antinociceptive, antidiarrheal, antimicrobial [6], and cytotoxic activities active against cancer cell lines with IC50 values of 1.66–20.49 μmol/L of different parts, mainly leaf extracts of C. indicum [10, 13–16].
Moreover, Ghosh and Pal [17, 18] explored the unique features in seedling flowering, stigma movement, plant phenology, and germination of C. indicum. They reported the plant as threatened in West Bengal, India, due to critical, unusual epigeal–cryptocotylar type of seedling and deceit pollination. Apart from that, the other causes are loss of habitat, massive exploitation of its medicinal values, and insect pest attack [19; https://indiabiodiversity.org/species/show/229224]. Clerodendrum species are listed by the International Union of Conservation of Nature (IUCN 2015; https://www.iucnredlist.org/), and several of these are either endangered, critically endangered, or vulnerable status [https://indiabiodiversity.org/species/show/229224].
The plant–microbe interactions in the rhizosphere are primary determinants for plant colonization and successful land acquisition in several abundant soil types [20, 21]. Among these, arbuscular mycorrhizas are the most important microbial symbioses that benefit the plants under various environmental conditions [22]. They form mutualistic associations with the plants in most terrestrial ecosystems [21, 23]. Through this association, plants facilitate nutrient uptake from the soil via an extensive AMF extraradical mycelium [24]. They contribute significantly to increase plant productivity by improving phosphorus (P) uptake [25], improving soil structure [26], enhancing pathogen resistance [27], and resisting toxic stresses [22, 28, 29]. Because of their importance in terrestrial ecosystems, AMF communities have been extensively studied both in natural [24–26] and in agricultural settings [27–29]. The studies by Fuchs and Haselwandter [30], Bothe et al. [31], and Radhika and Rodrigues [32], reported that the AM fungal diversity varies from the different localities of medicinal plants with a variation in the per cent colonization and spore density. They emphasized that medicinal plants could be restored from extinction by selective colonizing potential AM fungi and re-planting them into native sites. However, the mycorrhizal association on the Clerodendrum spp. host has not been well documented. For example, Gupta and Das [33] from Orissa State, India, observed 90% colonisation of mycorrhizae on C. inerme, while C. thomsonae had 40–60% root colonization. Kalita et al. [34] from Assam State, India, reported that Glomus is the most abundant vesicular arbuscular mycorrhizae (VAM) fungi in the rhizosphere of Clerodendron sp. Ghanta et al. [35] from West Bengal State, India, reported that in C. indicum the arbuscular frequency (0.1 to 31.7) and vesicular frequency (31.83 to 40.2) were the lowest as compared to the other medicinal plants tested. They also reported that the associated AM fungi belong to the species of Glomus. Therefore, these studies reflect that the assessment of mycorrhizal association in the Clerodendrum species is very random and no focus has been given to the determining factors that influence such variations. Thus, this study aims to assess the AM association in the different ecotypes of C. indicum grown in their native soil and to determine the soil parameters influencing the AM association.
Materials and methods
Sample collection
A total of 30 samples were collected from 10 different locations throughout West Bengal, India (Fig. S1). Three plant samples, root samples, and corresponding rhizospheric soil samples were collected. Soil samples were collected using a core sampler. Live plant samples were collected in plastic bags for further maintenance in the laboratory. Root samples were collected and immediately transferred to the icebox and then stored at −20 °C within 6 h for subsequent staining and DNA extraction. Sampling was done between February and April 2019. The ecotypes were maintained as the live culture at the Department of Botany, University of Gour Banga, West Bengal, India. The leaf morphology, phyllotaxy, and stem anatomy of the ecotypes were studied under dissecting (Magnus, Model Mag Star EM-200, Olympus Opto Systems India Pvt. Ltd, India) equipped with an Olympus camera (DX-500, 5MP) and phase-contrast microscope (Leica DM750, fitted with Leica ICC50W camera and LAS-EZ software, Germany) to distinguish the morphological peculiarities of the ecotypes. The geographical details of the locations of all the ecotypes are given in Table S1.
Assessment of mycorrhizal association in roots
For studying mycorrhizal association in roots, samples were prepared according to Sharma et al. [36], and AMF colonization was studied. Briefly, approximately 2-cm-length root samples from the root’s tip were taken and washed with double-distilled water to remove adhering particles from the root surface. The samples were macerated in 10% KOH (w/v) at 100 °C for 20 min, stained with 0.05% trypan blue in lactophenol (w/v) [37]. The association was observed under a phase-contrast microscope (DM750 Leica fitted with Leica ICC50W camera and software LAS-EZ, Germany). The degree of association, stages of association, and relative abundance correlated with the soil parameters according to the standard protocol [36, 38].
Determination of molecular diversity of mycorrhizae in roots
DNA extraction and gel electrophoresis
For molecular identification of AMF, all the root samples (~ 500 mg) were pooled in a 1.5-ml Eppendorf tube with a small number of glass beads (Sigma, USA) homogenized with a micro pestle. After that, 200 μl of extraction buffer {comprising 0.2 g (w/v) hexadecyltrimethylammonium bromide (CTAB, Sigma, USA), 1.0 ml of 100 mM Tris (pH 8.0) buffer (v/v), 0.1 ml of 20 mM EDTA (v/v), 0.82 g (w/v) NaCl, 0.4 g polyvinylpyrrolidone (PVP) (w/v), 0.01 g ascorbic acid (w/v), 0.007 ml of 10 mM β-mercaptoethanol (BME) (v/v), 0.1 g sodium dodecyl sulphate (SDS, w/v), and the volume was made to 10.0 ml by distilled water} was added and again homogenized [39]. After homogenization, 200 μl extraction buffer was added. The hard tissues were completely lysed with additional 1% sodium dodecyl sulfate (SDS, w/v) in the modified extraction buffer. After that, 10 μl lysozyme (10 mg/ml) was added and incubated at 65 °C for 1 h in a water bath. One microliter of proteinase K (10 mg/ml) was then added, mixed gently, and incubated at 37 °C for 1 h. An equal volume of phenol–chloroform (1:1; v/v) was added and mixed well by gentle inversion. The preparation was kept for 5 min. The tube was centrifuged at 12,851×g for 15 min, and the aqueous phase was taken into an Eppendorf tube. This step was repeated once if the aqueous phase was too much pigmented. After depigmentation, 0.6 volume of isopropanol was added to the aqueous portion and inverted twice and incubated for 20 min at 4 °C. The mixture was then centrifuged at 12,851×g for 15 min, and the supernatant was discarded. The pellet was then washed with chilled absolute ethanol and was centrifuged at 12,851×g for 5 min. After that, the supernatant was discarded, and the pellet (DNA) was suspended in 50 μl deionized water and incubated with 1 μl RNase (20 mg/ml) for 30 min at 37 °C to remove the RNAs. The DNA was kept at −20 °C for further use. The isolated genomic DNA was observed in 1% agarose gel electrophoresis [40].
PCR amplification, DNA sequencing, and phylogenetic analysis
The PCR amplification of AMF-specific short ribosomal subunit (SSU) regions was done using forward primer NS1 (5′ GTA GTC ATA TGC TTG TCT C3′) and reverse primer NS4 (5′ CTT CCG TCA ATT CCT TTAAG3′) (Sigma, USA) [41–43]. The total volume of the PCR mixture was 51 μl comprising of 46 μl 1× PCR master mix (Invitrogen, USA), 2 μl of 10 μmol primer pair each, 0.5 μl bovine serum albumin (BSA, 30 μg/ml), and 1 μl template DNA. The PCR reaction was performed in a thermal cycler (Applied Biosystems, USA) using initial denaturation at 95 °C for 3 min followed by 40 cycles of denaturation at 95 °C for 45 s, annealing at 44.5 °C for 30 s, extension at 72 °C for 1 min and a final extension of 72 °C for 4 min. The primary PCR product was re-amplified by different AMF genus-specific primers such as Acaulospora, Archeospora, Gigaspora, and Glomus (Table 1). The PCR initial denaturation was set at 95 °C for 3 min followed by 40 cycles of denaturation at 95 °C for 30 s, extension at 72 °C for 4 min, and a final extension of 72 °C for 1 min with primer-specific annealing temperatures. The amplicons were sequenced through Sanger’s method, and sequences were submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank/). BLAST was performed through the NCBI database. The phylogenetic tree was constructed based on the maximum likelihood method [44]. The nucleotide sequences were aligned in MEGA 6 software using the ClustalW parameter [45]. A maximum likelihood phylogenetic tree was constructed based on the best model selection for the provided nucleotide sequences, i.e., the Jukes-Cantor model with a gamma distribution pattern [46].
Table 1.
Primer used for the amplification of AMF
| Sl no. | Name and sequences of primers | Targeted genus | Annealing temperatures (°C) | References |
|---|---|---|---|---|
| 1. | NS1 (5′ GTA GTC ATA TGC TTG TCT C-3′) | Short ribosomal subunit (SSU) | 44.0 | Redecker [41]; Lee et al. [42]; Goswami et al. [43] |
| NS4 (5′ CTT CCG TCA ATT CCT TTA AG-3′) | ||||
| 2. | GLOM 1310F (5′AGC TAG GYC TAA CAT TGT TA) | Glomus sp. | 43.5 | |
| GLOM 5.8R (5′ TCC GTT GTT GAA AGT GAT C) | ||||
| 3. | GIGA5.8R (5′ ACT GAC CCT CAA GCA KGTG) | Gigaspora sp. | 52.0 | |
| ITS1F (5′ CTT GGT CAT TTA GAG GAA GTA A) | ||||
| 4. | ARCH1311F (5′ TGC TAA ATA GCC AGC CTG Y) | Archaeospora sp. | 53.0 | |
| ITS4R (5′ CAG ACT T(G/A)TA(C/T)ATG GTC CAG) | ||||
| 5. | ACAU1660F (5′ TGA GAC TCG GAT CGG) | Acaulospora sp. | 51.5 | |
| ITS4R (5′ CAG ACT T(G/A)TA(C/T)ATG GTC CAG) | ||||
| 6. | LETC1670F (5′ GAT CGG CGA TCG GTG AGT) | Glomeromycota | 53.0 | |
| ITS4R (5′ CAG ACT T(G/A)TA(C/T)ATG GTC CAG) |
Assessment of soil nutritional elements
Rhizospheric soil parameters, viz. pH, electrical conductivity, organic carbon, nitrogen, phosphorus, and potassium contents were measured according to the standard protocol [47, 48]. Briefly, pH and electric conductivity were measured by pH (Eutech, Germany) and electric conductivity (HPG, India) meter. Organic carbon was measured by the titration method where the soil sample was ground with potassium dichromate and ferrous ammonium sulphate solution used as the titer. Total nitrogen determination was done by the modified Kjeldahl method. The total content of the elemental phosphorus in soils was extracted and determined by perchloric acid digestion followed by spectrophotometric determination and total potassium in soils or minerals was best determined by decomposition of the sample employing hydrogen fluoride, followed by flame photometric determination. Measurements were carried out three times, and the average value was taken for further analysis.
Statistical analysis
An exploratory analysis [49] was done to get an overview of the data. The normality of the data was checked by the Shapiro–Wilk test, and Kruskal–Wallis rank-sum test was used for finding the variation significance in the data. Spearman’s rank correlation coefficient test was conducted for assessing the correlation between parameters. Principal component analysis (PCA) was conducted to deduce the overall correlation between all parameters [50]. Response surface methodology (RSM) was used for assessing interaction effects. A total of 12 RSM models were made with first-order, second-order, and two-way interaction terms. Arbuscule % was the response variable for six models, and Vesicle % was the response variable for another 6. The same permutation combination of predictor variables (soil parameters) was used for both the response variables; models (one for each response variable) with the lowest lack-of-fit value were selected for subsequent analysis and plotting [51]. All the analysis was done, and plots were made in R 3.6.3 software (www.r-project.org), and the analyses were run in the software environment RStudio 1.2.5042 (http://www.rstudio.com). Shapiro–Wilk test, Kruskal–Wallis rank-sum test, Spearman’s rank correlation coefficient test, and PCA base functions of R were used. For making RSM models, the “rsm” package was used. For plotting “ggplot2,” “ggpubr,” “factoextra,” “ggrepel,” “corrplot,” and base plotting functions were used.
Results
Morphology of the C. indicum ecotypes
The ecotypes (Fig. S2) possessed distinguishable variations in the leaf morphology, phyllotaxy, and stem anatomy (Fig. S3). Variation and their combination in leaf shape found were acute (Fig. S3, b1 to b6), apiculate (Fig. S3, b5, b7), cuneate (b1), elliptic (b2), lanceolate (Fig. S3, b4, and b6), linear (Fig. S3, b7, and b8), and oblanceolate (Fig. S3, b1, b3, and b7). In the leaf edge, all were found with an entire (Fig. S3, b1, b2, b3, b5, b6, b7, and b8) edge, except one which was found with a serrate (b4) edge. Leaves were arranged in whorls of two to six leaves (Fig. S3, a to d, f, g, a1 to a4). The stem has ridges and furrows, and the number corresponds to the number of leaves in the whorl. The mature stem was hollow throughout (Fig. S3, f, g, a1 to a4); the hollow core was narrow at the node and wide at the internode (Fig. S3, a1 to a4). Near the node, a thin layer of tissue was found that separates two internode sections (Fig. S3, a1 to a4). The young stem was not hollow and was filled with tissue (Fig. S3, a to e). A summarized morphological description of ecotypes is given in Table S2.
Microscopic assessment of mycorrhizal association in roots
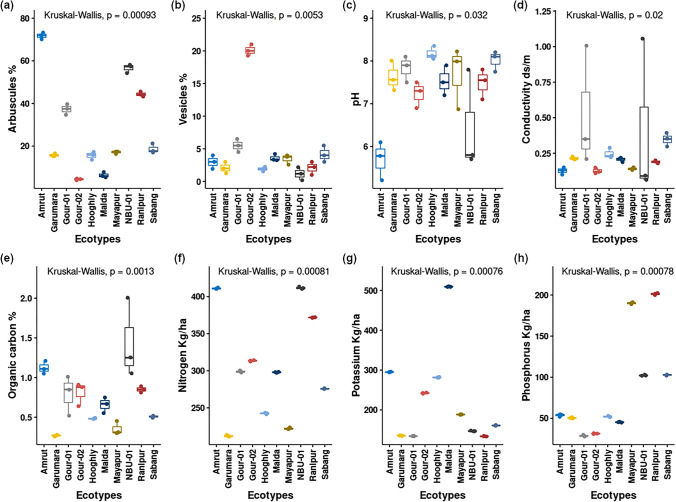
The examination of mycorrhizal association in the roots of different ecotypes of C. indicum (Fig. S4) showed that all the ecotypes have mycorrhizal associations with different forms/stages like hyphae, arbuscules, and vesicles in the roots (Fig. S4). According to INVAM (INVAM, 2017, http://Fungi.Invam.Wvu.Edu/the-Fungi/Species-Descriptions.html) and TERI (http://mycorrhizae.org.in/cmcc/) (TERI, 2018, https://www.teriin.org/article/fungi-central-mycorrhiza-haven), morphological descriptions of the infested AMF were found morphologically similar to Glomus sp. Mycelial associations were found both in the apoplastic and symplastic regions of the cortex of young roots [52]. Microscopic assessment of root length AMF colonization data of ecotypes (Amrut, Garumara, Gour-01, Gour-2, Hooghly, Malda, Mayapur, NBU-01, Ranipur, and Sabang) showed that there is a significant difference in the formation of arbuscules (p = 0.00093) and vesicles (p = 0.0053) between ecotypes (Fig. 1a, b).
Fig. 1.
Box plots showing data distribution and variation in Arbuscule % (a), Vesicle % (b), pH (c), electrical conductivity (d), Organic carbon % (e), nitrogen (f), potassium (g), and phosphorus (h) of the host ecotypes
Molecular diversity assessment of AMF
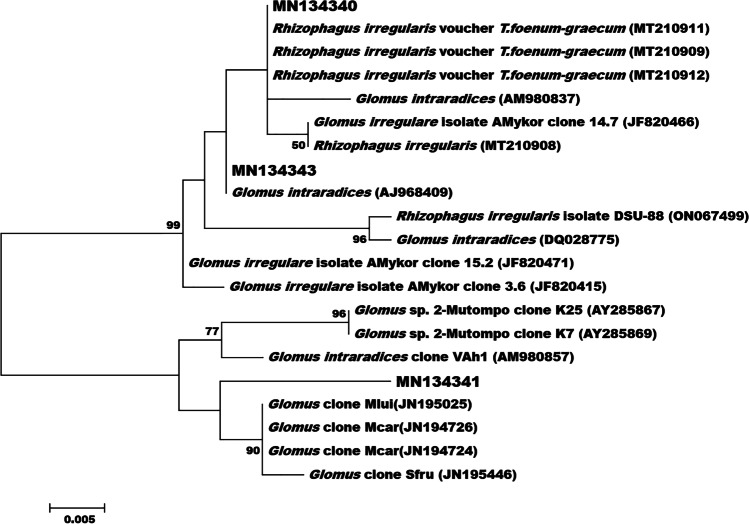
The molecular identification of AM fungi through ITS and NS1 and NS4, genus-specific primer sequences, and the phylogenetic tree show that the amplicon sequences of the AMF were closely related to R. irregularis (GenBank Ac. MN134340) and G. intraradices (GenBank Ac. No. MN134341 and MN134343) (Fig. 2).
Fig. 2.
Phylogenetic tree was prepared by the maximum likelihood method. Amplicon sequences of this study are marked with the GenBank Ac. No. MN134340, MN134341, MN134343.
Analysis of VAM and soil parameters
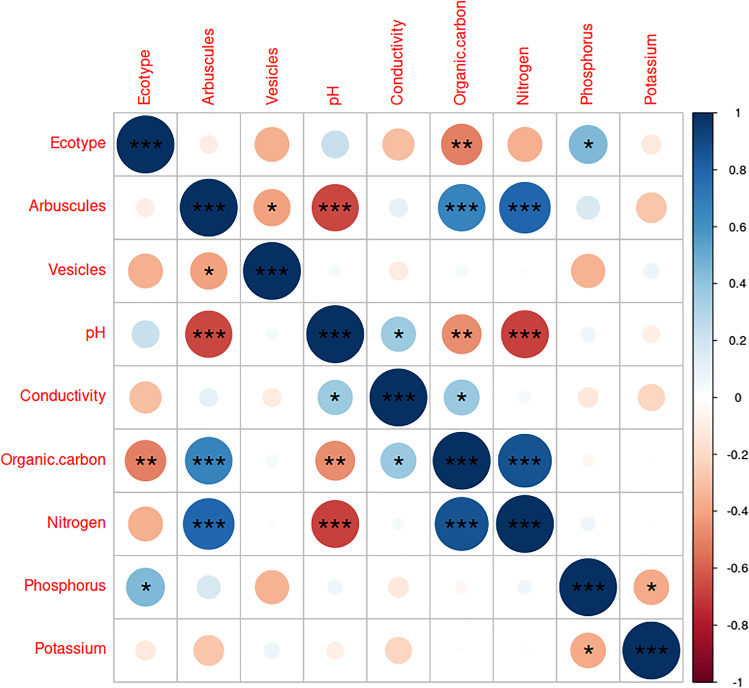
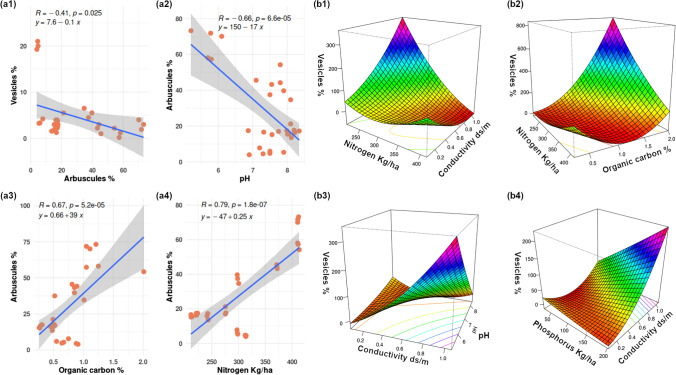
From the Kruskal–Wallis test, a significant variation of soil parameters pH (df = 9, p-value = 0.03183), conductivity (df = 9, p-value = 0.02033), organic carbon (df = 9, p-value = 0.001301), nitrogen (df = 9, p-value = 0.0008128), phosphorus (df = 9, p-value = 0.0007833), and potassium (df = 9, p-value = 0.0007644) was found between the ecotypes (Fig. 1c–h). The correlation matrix (Fig. 3) created with Spearman’s rank correlation coefficient test indicated that arbuscule and vesicles are negatively correlated, and the correlation was found significant (R = −0.41, p = 0.025). The arbuscule and pH are negatively correlated, and the relation was found highly significant (R = −0.66, p = 6.6e−05); arbuscule and conductivity were found more or less negatively correlated, but the relation was not found significant (R = −0.11, p = 0.57).
Fig. 3.
Correlation matrix showing the correlation between all the parameters, the scale indicate positive (+1) and negative (−1) correlations, significance of the correlation between parameters is indicated by stars (“*”) mark (***p < 0.001, **p < 0.01, *p < 0.05)
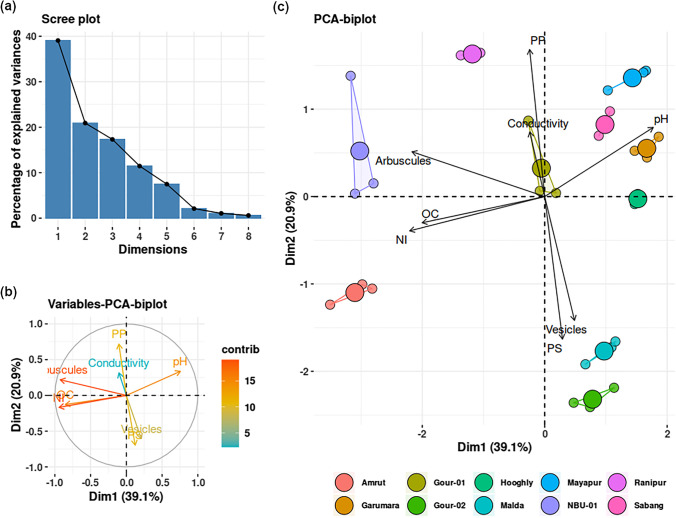
From the principal component analysis (PCA), the scree plot (Fig. 4a) showed that components 1 and 2 explained more than 50% variation in the data. It was observed from the biplots that arbuscule percentage, organic carbon, and nitrogen content contributed most to data variation, and conductivity contributed the least (Fig. 4b). Overall, two groups of the parameters could be made, in which parameters are positively correlated within the group and negatively correlated between the groups. Group 1 comprises arbuscule, organic carbon, nitrogen, phosphorus, and electric conductivity, and group 2 comprises vesicle, pH, and potassium (Fig. 4c). For a better symbiotic impact on the host, the organic carbon and nitrogen content in soil could promote more arbuscule formation, while the factors influencing the soil conductivity have the least influence on host colonization and arbuscule formation, which indicates that the plant could survive in moderate to high salt-containing soils making it more adaptive in barren lands.
Fig. 4.
Scree plot (a) showing the percentage of explained variables by the principal components. Variable-PCA-biplot (b) showing the contribution of variables in data variation. Individual-PCA-biplot (c) showing the overall correlation of the parameters (grouped by the ecotypes)
From the response surface models, the selected model for assessing the interaction effects of the parameters on arbuscule formation (R2: 0.9673, adjusted R2: 0.8946, DF = 9, p-value: 0.000197) showed none of the interactions between parameters found significant, though significant direct correlations were evident (Fig. 5 a1 to a4). The selected model for assessing the interaction effects of parameters on vesicle formation (R2: 0.9631, adjusted R2: 0.8812, DF = 9, p-value: 0.0003283) showed that an interaction between conductivity: nitrogen (p = 0.0138952), conductivity: phosphorus (p = 0.0004532), organic-carbon: nitrogen (p = 0.0296641), pH: conductivity (p = 0.0122054) was significant (Fig. 5 b1 to b4). From the surface plots, it was observed that when the nitrogen content was low, conductivity had a great effect on vesicle formation, but at a higher concentration of nitrogen the effect of conductivity on vesicle formation was not high, while low nitrogen and high conductivity increased vesicle formation; however, high nitrogen and high conductivity reduced vesicle formation (Fig. 5 b1). When the nitrogen content was low, organic carbon greatly affected vesicle formation, but a higher concentration of nitrogen effect of organic carbon on vesicle formation was quite different from the previous; low nitrogen and high organic carbon increased vesicle formation. However, low nitrogen and low organic carbon reduced vesicle formation (Fig. 5 b2). Low pH and high conductivity greatly influenced vesicle formation. However, low pH–low conductivity greatly reduced vesicle formation; at lower conductivity, pH did not affect vesicle formation, but at higher conductivity, pH had great effects on vesicle formation (Fig. 5 b3). A higher concentration of phosphorus and lower conductivity greatly affected the vesicle formation, and also at higher conductivity, phosphorus greatly affected vesicle formation. High phosphorus and high conductivity greatly increased vesicle formation, and high phosphorus content with low conductivity greatly reduced vesicle formation (Fig. 5 b4). Hence, the RSM model also supports the results of the PCA analysis. This also emphasizes the observation of the percent arbuscule and vesicle formation in the varied soil nutritional parameters.
Fig. 5.
Scatter plots aided with linear regression line (a1 to a4) showing correlations between parameters and slope of the models, the shaded area around the blue regression line indicating the confidence (0.85) interval. Response surface plots (b1 to b4) aided by contours at the base of the plots, showing the interaction effect of parameters on vesicle formation
Discussions
Mycorrhizal symbioses between plant roots and soil fungi are ancient, having evolved with the earliest plants to colonize Earth’s land surfaces >400 Mya [53]. They provide essential nutritional constituents, phosphorus, and zinc to the associating plants [54]. However, due to human activities, with depletion of natural resources, land degradation, drop-in soil nutritional contents, and the changing climate have become a great concern for the naturally growing plants around us [55]. These extensive thrusts lead to many valuable medicinal plants to get extinct [55]. C. indicum is among them which has a multitude of life-supporting medicinal constituents in all its parts [56] and has obtained a threatened status due to these reasons [https://indiabiodiversity.org/species/show/229224]. Therefore, a thorough investigation of the habit and habitat conditions and soil parameters is of the utmost need for their eco-restoration [57, 58]. The present study deciphered that the host C. indicum harbours R. irregularis and G. intraradices as the associated AMF grown in different eco-geographical locations. However, the degree of association varied based on soil nutritional conditions. Song et al. [59] and Schalamuk et al. [60] reported that the AMF Glomus sp. and Acaulospora sp. were associated with the species C. colebrookianum and C. buchananii, respectively. This indicates that the AMF species association varies from species to species and also the eco-geographical variation of the host. A study by Jansa et al. [61] reported that plants could harbor more than one AMF species at a time, and this simultaneous root colonization by different AMF provides more P than a selective species. Thus, a thorough investigation is required from the different species of Clerodendrum from the same locality to establish the strict or relaxed biasness of the AMF association among the Clerodendrum spp.
Nouri et al. [62] previously reported that the mycorrhizal association does not directly depend on soil organic carbon or available potassium content. However, in this study, it was found that arbuscule and organic carbon are positively correlated (Figs. 3, 4, and 5) and the relation was found significant (R = 0.53, p = 0.0023). This is similar to the observations of Hodge and Storer [63] where they reported that the organic carbon accumulation in the host is due to a higher AM association in the root and soil environment [63]. While arbuscule and potassium were found negatively correlated, the relation was not found significant (R = −0.28, p = 0.14). Nitrogen and arbuscule were found positively correlated, and the relation was found significant (R = 0.55, p = 0.0018), probably because arbuscular mycorrhizae (AM) enhance nitrogen uptake [63]. Arbuscule and phosphorus were found positively correlated but not significant (R = 0.16, p = 0.38). The vesicle and nitrogen were found negatively correlated, but the relation was not found significant (R = −0.03, p = 0.84); vesicle and phosphorus were found negatively correlated, but the relation was not found significant (R = −0.34, p = 0.063); and vesicle and potassium were found positively correlated, but the relation was not found significant (R = 0.072, p = 0.71). Vesicle and pH were found to have a very minute positive correlation, but the relationship was not significant (R = 0.043, p = 0.82); vesicle and conductivity were found to be negatively correlated. The relation was not found significant (R = 0.097, p = 0.61); vesicle and organic carbon were found positively correlated; however, the relation was not significant (R = 0.05, p = 0.79). Thus, the study revealed that the physiological function of mycorrhizas and mycorrhiza-like symbioses is dependent upon several abiotic and biotic factors [19, 28, 29]. The result of soil analysis reveals that the rhizospheric soils deficient in nutrients might be suitable for mycorrhizal symbiosis with plants [64, 65]. It was also found that soils with higher amounts of sodium had reduced AM fungi and with higher calcium and organic matter had greater AM fungi with Glomerospore abundance [66].
It was also observed that among the species of AMF, the genera Glomus showed dominance in the plant root and rhizospheric soil of most medicinal plants and have wider host preferences to provide a greater allocation of nutrients [20, 22–28, 30–35]. Therefore, the successful restoration of native infective AMF can potentially improve the eco-restoration success of C. indicum in both arable and degraded lands. A better AMF inoculation with consortia of a few or single species could also result in species survival [61, 67, 68].
Conclusions
The study concludes that the ecotypes of C. indicum possess a distinguishable variation in the leaf morphology, phyllotaxy, and stem anatomy. The host species forms an association with R. irregularis and G. intraradices in different ecological conditions. However, the degree of AMF association with the hosts shows variations, like hyphae, arbuscules, and vesicles greatly depending on the soil parameters. Thus, through the selective mycorrhizae species association, a large-scale propagation could be undertaken for the conservation of this threatened medicinal plant and to protect it from extinction.
Supplementary information
(DOCX 3961 kb)
(DOCX 18 kb)
Acknowledgements
The authors are grateful to Mr. Indrajit Mandal (Research Scholar) and Professor Swadesh Pal, Department of Geography, University of Gour Banga, Malda-732103, W.B., India, for providing the geographical map of West Bengal. We are grateful to Dr. Biraj Sarkar (Research Scholar) and Dr. Sukhendu Mandal, Department of Microbiology, Calcutta University, for the phylogenetic tree preparation. We are grateful to the Soil testing laboratory, Govt. of West Bengal, English Bazar, Malda-732103 for soil nutritional parameters analysis. We are also grateful to Late Prof. Pankaj Kumar Pal, Department of Botany, The University of Burdwan, W.B., India for his valuable suggestion and guidance in this research work.
Availability of data and material
The data has been submitted as a supplementary compressed file along with this article.
Code availability
Not applicable
Database accession numbers
The GenBank Accession number of Glomus sp. clones of Clerosp_ Gour-01 is MN134340, that of Clerosp_ Sabang is MN134343, and that of Clerosp_ NBU-01 is MN134341. The Glomus sp. clones were marked along with GenBank Ac. No. as in the phylogenetic tree.
Author contribution
All authors contributed to the study’s conception and design. Prashanta Kumar Mitra, Rajsekhar Adhikary, and Ashutosh Kundu performed the material preparation, data collection, and analysis. Prashanta Kumar Mitra, Rajsekhar Adhikary, and Ashutosh Kundu wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization and design of experimentation: Vivekananda Mandal; methodology: Prashanta Kumar Mitra, Rajsekhar Adhikary, Prithwish Mandal, and Ashutosh Kundu did the experimentations; Prashanta Kumar Mitra did the statistical analysis and data analysis for its presentation; formal analysis and investigation: Prashanta Kumar Mitra, Rajsekhar Adhikary; writing — original draft preparation: Prashanta Kumar Mitra, Rajsekhar Adhikary. All the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; writing — review and editing: Vivekananda Mandal; supervision: Vivekananda Mandal; funding acquisition: Vivekananda Mandal.
Funding
This work was supported by the Department of Science and Technology, Government of West Bengal, for funding the project [Sanction letter No.: 285/(Sanc.)/ST/P/S&T/2G-10/2017, Dated: 28.03.2018] and by UGC-DAE-CRS (Sanction letter No.: UGC-DAE-CSR-KC/CRS/19/RB-02/1045/1063; Dated: 10.05.2019).
Declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Footnotes
Online documents
1.India Biodiversity Portal. https://indiabiodiversity.org/species/show/229224. Accessed 16 July 2022
2.The Plant List (TPL). http://www.theplantlist.org/. Accessed 16 July 2022
3.IUCN 2022. The IUCN Red List of Threatened Species. Version 2021-3. https://www.iucnredlist.org. Accessed 16 July 2022
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Prashanta Kumar Mitra, Email: pkmitrabot@gmail.com.
Rajsekhar Adhikary, Email: radhikary468@gmail.com.
Prithwish Mandal, Email: prithwish315@gmail.com.
Ashutosh Kundu, Email: ashubot93.ak@gmail.com.
Vivekananda Mandal, Email: mandalvivek@gmail.com, Email: vivek.bot@ugb.ac.in.
References
- 1.Taram M, Borah D, Tag H, Choudhary RK (2020) An inventory of the native flowering plants in East Siang District of Arunachal Pradesh, India. J Threatened Taxa 12(17):17299–17322. 10.11609/jott.6241.12.17.17299-17322
- 2.Srivastava N, Patel T. Clerodendrum and healthcare: an overview-part II phytochemistry and biotechnology. Med Aromat Plant Sci Biotechnol. 2007;1(2):209–223. [Google Scholar]
- 3.Wahba HM, AbouZid SF, Sleem AA, Apers S, Pieters L, Shahat AA. Chemical and biological investigation of some Clerodendrum species cultivated in Egypt. Pharm Biol. 2011;49(1):66–72. doi: 10.3109/13880209.2010.494674. [DOI] [PubMed] [Google Scholar]
- 4.Kyaw EH, Iwasaki A, Suenaga K, Kato-Noguchi H. Phytotoxic activity of Clerodendrum indicum (L.) Kuntze and its potential phytotoxic substance. Emir J Food Agric. 2021;33(10):884–892. [Google Scholar]
- 5.Sidde LS, Malathi S, Malathi SS (2018) A brief review on Clerodendrum indicum. Int J Indig Med Plants 31:1-4
- 6.Pal A, Mahmud ZA, Akter N, Islam S, Bachar SC. Evaluation of antinociceptive, antidiarrheal and antimicrobial activities of leaf extracts of Clerodendrum indicum. Pharmacogn J. 2012;4:41e46. doi: 10.5530/pj.2012.30.8. [DOI] [Google Scholar]
- 7.Majumder S, Nahar T, Mahmud S. Investigation on in vitro antioxidant and in vivo neurobehavioral activities of Clerodendrum indicum leaf extract. Biores Com (BRC). 2019;5(2):770–781. [Google Scholar]
- 8.Tian J, Zhao QS, Zhang HJ, Lin ZW, Sun HD. New cleroindicins from Clerodendrum indicum. J Nat Prod. 1997;60(8):766–769. doi: 10.1021/np9606759. [DOI] [Google Scholar]
- 9.Jun T, Handong S. Chemical constituents of Clerodendrum indicum. Nat Prod Res Develop. 1999;11(3):1–5. [Google Scholar]
- 10.Ravindranath N, Ramesh C, Kishore KH, Murty USN, Das B (2003) Clerodendrone, a novel hydroquinone diterpenoid from Clerodendrum indicum. J Chem Res 2003, 440-441. 10.3184/2F030823403103174452
- 11.Wang JH, Luan F, He XD, Wang Y, Li MX. Traditional uses and pharmacological properties of Clerodendrum phytochemicals. J Trad Complement Med. 2018;8(1):24–38. doi: 10.1016/j.jtcme.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sushma M, Lahari S, Mounika A, Sailaja KE. Phytochemical screening & in-vitro evaluation of anti-inflammatory activity of Clerodendrum indicum roots. World J Curr Med Pharm Res. 2021;28:140–143. doi: 10.37022/wjcmpr.v3i6.201. [DOI] [Google Scholar]
- 13.Gogoi B, Gogoi D, Silla Y, Kakoti BB, Bhau BS. Network pharmacology-based virtual screening of natural products from Clerodendrum species for identification of novel anti-cancer therapeutics. Mol BioSyst. 2017;13(2):406–416. doi: 10.1039/C6MB00807K. [DOI] [PubMed] [Google Scholar]
- 14.Somwong P, Suttisri R. Cytotoxic activity of the chemical constituents of Clerodendrum indicum and Clerodendrum villosum roots. J Integr Med. 2018;16(1):57–61. doi: 10.1016/j.joim.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Kar P, Dutta S, Chakraborty AK, Roy A, Sen S, Kumar A, Lee J, Chaudhuri TK, Sen A. The antioxidant-rich active principles of Clerodendrum sp. controls haloalkane xenobiotic induced hepatic damage in the murine model. Saudi. J Biol Sci. 2019;26(7):1539–1547. doi: 10.1016/j.sjbs.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priya K, Setty MM, Pai K. In vitro and in vivo evaluation of anticancer properties of Clerodendrum indicum (L.) Kuntze in colon cancer. Res J Pharm Technol. 2020;13(5):2321–2328. doi: 10.5958/0974-360X.2020.00418.7. [DOI] [Google Scholar]
- 17.Ghosh A, Pal PK. Seedling phenology of Clerodendrum indicum exhibiting the unusual epigeal cryptocotylar type of germination. Acta Bot Gall. 2015;162(3):233–237. doi: 10.1080/12538078.2015.1023218. [DOI] [Google Scholar]
- 18.Ghosh A, Pal PK. Pollination ecology of Clerodendrum indicum (Lamiaceae): first report of deceit pollination by anther-mimicking stigma in a bisexual flower. Rev Biol Trop. 2017;65(3):988. doi: 10.15517/rbt.v65i3.29450. [DOI] [Google Scholar]
- 19.Borkataki SH, Das PU, Deka RL, Borua IC, Saud BK, Sharma A. Influence of weather factors on incidence and intensity of Black inchworm (Hyposidra talaca Walker) on Clerodendrum indicum (L.) Kuntze at Jorhat. J Agrometeorol. 2018;20:98–101. [Google Scholar]
- 20.Morgan JA, Bending GD, White PJ. Biological costs and benefits to plant–microbe interactions in the rhizosphere. Journal of Experimental Botany. 2005;56(417):1729–1739. doi: 10.1093/jxb/eri205. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys CP, Franks PJ, Rees M, Bidartondo MI, Leake JR, Beerling DJ. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nat Commun. 2010;1(1):1–7. doi: 10.1038/ncomms1105. [DOI] [PubMed] [Google Scholar]
- 22.Jeffries P, Gianinazzi S, Perotto S, et al. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils. 2003;37:1–16. doi: 10.1007/s00374-002-0546-5. [DOI] [Google Scholar]
- 23.Martin FM, Uroz S, Barker DG. Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science. 2017;356(6340):eaad4501. doi: 10.1126/science.aad4501. [DOI] [PubMed] [Google Scholar]
- 24.Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol. 2010;12:2165–2179. doi: 10.1111/j.1462-2920.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 25.Lekberg Y, Schnoor T, Kjøller R, Gibbons SM, Hansen LH, Al-Soud WA, Sorensen SJ, Rosendahl S. 454-Sequencing reveals stochastic local reassembly and high disturbance tolerance within arbuscular mycorrhizal fungal communities. J Ecol. 2012;100:151–160. doi: 10.1111/j.1365-2745.2011.01894.x. [DOI] [Google Scholar]
- 26.Xiang D, Verbruggen E, Hu Y, Veresoglou SD, Rillig MC, Zhou W, Xu T, Li H, Hao Z, Chen Y, Chen B. Land use influences arbuscular mycorrhizal fungal communities in the farming-pastoral ecotone of Northern China. New Phytol. 2014;204:968–978. doi: 10.1111/nph.12961. [DOI] [PubMed] [Google Scholar]
- 27.Gosling P, Mead A, Proctor M, Hammond JP, Bending GD. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol. 2013;198:546–556. doi: 10.1111/nph.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bainard LD, Bainard JD, Hamel C, Gan Y. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. FEMS Microbiol Ecol. 2014;88:333–344. doi: 10.1111/1574-6941.12300. [DOI] [PubMed] [Google Scholar]
- 29.Higo M, Isobe K, Drijber RA, Kondo K, Yamaguchi M, Takeyama S, Suzuki Y, Niijima D, Matsuda Y, Ishii R, Torigoe Y. Impact of a 5-year winter cover crop rotational system on the molecular diversity of arbuscular mycorrhizal fungi colonizing roots of subsequent Soybean. Biol Fertil Soils. 2014;50:913–926. doi: 10.1007/s00374-014-0912-0. [DOI] [Google Scholar]
- 30.Fuchs B, Haselwandter K (2008) Arbuscular mycorrhiza of endangered plant species: potential impacts on restoration strategies. In: Varma, A. (eds) Mycorrhiza. Springer, Berlin, Heidelberg. 10.1007/978-3-540-78826-3_27
- 31.Bothe H, Turnau K, Regvar M. The potential role of arbuscular mycorrhizal fungi in protecting endangered plants and habitats. Mycorrhiza. 2010;20:445–457. doi: 10.1007/s00572-010-0332-4. [DOI] [PubMed] [Google Scholar]
- 32.Radhika KP, Rodrigues BF. Arbuscular mycorrhizal fungal diversity in some commonly occurring medicinal plants of the Western Ghats, Goa region. J For Res. 2010;21:45–52. doi: 10.1007/s11676-010-0007-1. [DOI] [Google Scholar]
- 33.Gupta N, Das P. Study on arbuscular mycorrhizal associations in ornamental plants—a survey. J Phytol Res. 2001;14:171–174. [Google Scholar]
- 34.Kalita RK, Bora DP, Dutta D. Vesicular arbuscular mycorrhizal association with some native plants. Ind J Fores. 2002;25(1/2):143–146. [Google Scholar]
- 35.Ghanta R, Dutta S, Mukhopadhyay R. Investigation on arbuscular mycorrhizal alliances in some threatened medicinal herbs of Burdwan district, West Bengal. India. J Med Plants Res. 2013;7(7):315–323. [Google Scholar]
- 36.Sharma C, Gupta RK, Pathak RK, Choudhary KK. Seasonal colonization of arbuscular mycorrhiza fungi in the roots of Camellia sinensis (Tea) in different tea gardens of India. ISRN Biodiversity. 2013;2013:1–6. doi: 10.1155/2013/593087. [DOI] [Google Scholar]
- 37.Phillip JM, Hayman DS. Improved procedure for clearing roots and staining parasites and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158–160. doi: 10.1016/S0007-1536(70)80110-3. [DOI] [Google Scholar]
- 38.Brundrett MC, Bougher N, Dell B, Grove T, Malajczuk N (Editors) (1996) Working with mycorrhizas in forestry and agriculture. 374p. ACIAR Monograph 32. Australian Centre for International Agricultural Research, Canberra, Australia
- 39.Keb-Llanes M, González G, Chi-Manzanero B, Infante D. A rapid and simple method for small-scale DNA extraction in Agavaceae and other tropical plants. Plant Mol Biol Rep. 2002;20(3):299–299. doi: 10.1007/bf02782465. [DOI] [Google Scholar]
- 40.Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual. 2nd Edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
- 41.Redecker D. Specific PCR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza. 2000;10:73–80. doi: 10.1007/s005720000061. [DOI] [Google Scholar]
- 42.Lee J, Lee S, Young JP. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol. 2008;65(2):339–349. doi: 10.1111/j.1574-6941.2008.00531.x. [DOI] [PubMed] [Google Scholar]
- 43.Goswami BR, Parakhia MV, Golakiya BA, Kothari CR. Morphological and molecular identification of Arbuscular Mycorrhizal (AM) fungi. Int J Curr Microbiol App Sci. 2018;7(01):2336–2347. doi: 10.20546/ijcmas.2018.701.282. [DOI] [Google Scholar]
- 44.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 45.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jukes TH, Cantor CR. Evolution of protein molecules. New York: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 47.Sarkar DK, Haldar A. Physical and chemical methods of soil analysis. New Age International Publishers; 2005. [Google Scholar]
- 48.Sparks DL, Page AL, Helmke PA, Loeppert RH, editors. Methods of soil analysis. Part 3. Chemical methods. Madison, Wisconsin: Soil Science Society of America; 1996. [Google Scholar]
- 49.Datar R, Garg H (2019) Hands-on exploratory data analysis with R. Packt Publishing. https://www.packtpub.com/product/hands-on-exploratory-data-analysis-with-r/9781789804379
- 50.Zelterman D (2015) Applied multivariate statistics with R. Springer Publishing. 10.1007/978-3-319-14093-3
- 51.Lenth RV. Response-surface methods inR, Usingrsm. Journal of Statistical Software. 2009;32(7):1–21. doi: 10.18637/jss.v032.i07. [DOI] [Google Scholar]
- 52.Van der Heijden MGA, Martin FM, Selosse MA, Sanders IR. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 2015;205(4):1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- 53.Remy W, Taylor TN, Hass H, Kerp H. Four hundred-million-year-old vesicular-arbuscular mycorrhizae. Proc Natl Acad Sci USA. 1994;91(25):11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finlay RD. Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot. 2008;59(5):1115–1126. doi: 10.1093/jxb/ern059. [DOI] [PubMed] [Google Scholar]
- 55.Kala CP, Dhyani PP, Sajwan BS. Developing the medicinal plants' sector in northern India: challenges and opportunities. J Ethnobiol Ethnomedicine. 2006;2(1):1–5. doi: 10.1186/1746-4269-2-32. [DOI] [Google Scholar]
- 56.van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396: 69-72. http://dx.doi.org/10.1038/23932
- 57.Ghosh A, Kumar RV, Manna MC, Singh AK, Parihar CM, Kumar S, Roy AK, Koli P. Eco-restoration of degraded lands through trees and grasses improve soil carbon sequestration and biological activity in tropical climates. Ecol Eng. 2021;162:106176. doi: 10.1016/j.ecoleng.2021.106176. [DOI] [Google Scholar]
- 58.Johnson NC, Angelard C, Sanders IR, Kiers ET. Predicting community and ecosystem outcomes of mycorrhizal responses to global change. Ecol Lett. 2013;16:140–153. doi: 10.1111/ele.12085. [DOI] [PubMed] [Google Scholar]
- 59.Song J, Han Y, Bai B, Jin S, He Q, Ren J. Diversity of arbuscular mycorrhizal fungi in rhizosphere soils of the Chinese medicinal herb Sophora flavescens Ait. Soil Tillage Res. 2019;195:104423. doi: 10.1016/j.still.2019.104423. [DOI] [Google Scholar]
- 60.Schalamuk S, Velazquez S, Chidichimo H, Cabello M. Fungal spore diversity of arbuscular mycorrhizal fungi associated with spring wheat: effects of tillage. Mycologia. 2006;98(1):16–22. doi: 10.1080/15572536.2006.11832708. [DOI] [PubMed] [Google Scholar]
- 61.Jansa J, Smith FA, Smith SE. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008;177(3):779–789. doi: 10.1111/j.1469-8137.2007.02294.x. [DOI] [PubMed] [Google Scholar]
- 62.Nouri E, Breuillin-Sessoms F, Feller U, Reinhardt D (2015) Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS One 10(4):e0127472. 10.1371/journal.pone.0127472 [DOI] [PMC free article] [PubMed]
- 63.Hodge A, Storer K. Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil. 2015;386:1–19. doi: 10.1007/s11104-014-2162-1. [DOI] [Google Scholar]
- 64.Zubek S, Błaszkowski J. Medicinal plants as hosts of arbuscular mycorrhizal fungi and dark septate endophytes. Phytochem Rev. 2009;8(3):571–580. doi: 10.1007/s11101-009-9135-7. [DOI] [Google Scholar]
- 65.Islam M, Al-Hashimi A, Ayshasiddeka M, Ali H, El Enshasy HA, Dailin DJ, Sayyed RZ, Yeasmin T. Prevalence of mycorrhizae in host plants and rhizosphere soil: a biodiversity aspect. PLoS One. 2022;17(3):e0266403. doi: 10.1371/journal.pone.0266403. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Medeiros AS, Goto BT, Ganade G. Ecological restoration methods influence the structure of arbuscular mycorrhizal fungal communities in degraded drylands. Pedobiol. 2021;84:150690. doi: 10.1016/j.pedobi.2020.150690. [DOI] [Google Scholar]
- 67.Asmelash F, Bekele T, Birhane E. The potential role of arbuscular mycorrhizal fungi in the restoration of degraded lands. Front Microbiol. 2016;7:1095. doi: 10.3389/fmicb.2016.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thirkell TJ, Charters MD, Elliott AJ, Sait SM, Field KJ. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J Ecol. 2017;105(4):921–929. doi: 10.1111/1365-2745.12788. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 3961 kb)
(DOCX 18 kb)
Data Availability Statement
The data has been submitted as a supplementary compressed file along with this article.