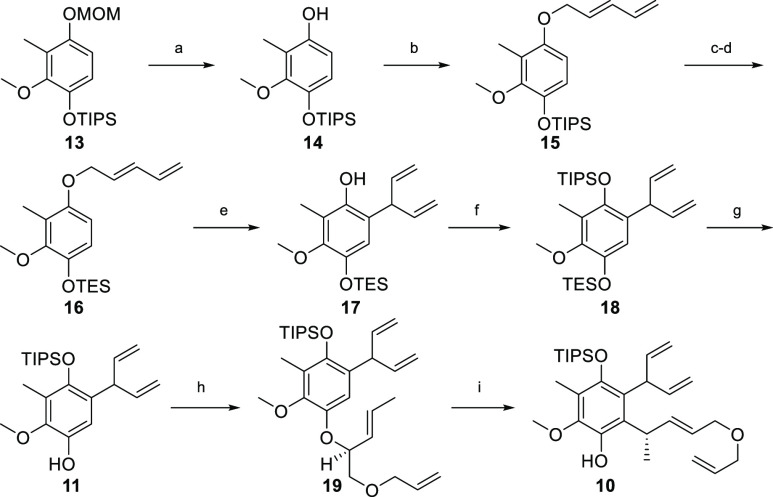
Scheme 2. Synthesis of Compound 10.
Reagents and conditions: (a) ZnBr2, EtSH, DCM, −32 to −5 °C, 2.5 h, 89%; (b) n-BuLi, (E)-5-bromopenta-1,3-diene, 1.4:1 THF/DMF, −35 °C to rt, 15 h, 97%; (c) TBAF, THF, rt, 2 min, 95%; (d) TESCl, imidazole, DCM, rt, 20 min, 95%; (e) EuFOD, PhMe, 110 °C, 4 h, 84%; (f) n-BuLi, TIPSOTf, THF, −85 to −45 °C, 1.5 h, 92%; (g) TFA, 4:1 THF/H2O, rt, 30 min, 93%; (h) PBu3, ADDP, 12, PhMe, 0 °C to rt, 2 h, 69%; (i) EuFOD, o-xylene, 120 °C, 18 h, 55% (+31% 11).