Abstract
Introduction
Despite important advances in many areas of hepatobiliary surgical practice during the past decades, posthepatectomy liver failure (PHLF) still represents an important clinical challenge for the hepatobiliary surgeon. The aim of this review is to present the current body of evidence regarding different aspects of PHLF.
Methods
A literature review was conducted to identify relevant articles for each topic of PHLF covered in this review. The literature search was performed using Medical Subject Heading terms on PubMed for articles on PHLF in English until May 2022.
Results
Uniform reporting on PHLF is lacking due to the use of various definitions in the literature. There is no consensus on optimal preoperative assessment before major hepatectomy to avoid PHLF, although many try to estimate future liver remnant function. Once PHLF occurs, there is still no effective treatment, except liver transplantation, where the reported experience is limited.
Discussion
Strict adherence to one definition is advised when reporting data on PHLF. The use of the International Study Group of Liver Surgery criteria of PHLF is recommended. There is still no widespread established method for future liver remnant function assessment. Liver transplantation is currently the only effective way to treat severe, intractable PHLF, but for many indications, this treatment is not available in most countries.
The aim of this review is to present the current body of evidence regarding different aspects of posthepatectomy liver failure (PHLF). There is no consensus on optimal preoperative assessment before major hepatectomy to avoid PHLF, although many try to estimate future liver remnant function. Strict adherence to one definition is advised when reporting data on PHLF.
Introduction
Despite important advances in many areas of hepatobiliary surgical practice during the last decades, posthepatectomy liver failure (PHLF) still represents an important clinical challenge for the hepatobiliary surgeon. Even if clinically relevant PHLF is not the most common complication after a liver resection, it continues to be the leading cause for postoperative fatalities even in modern practice at tertiary centres1,2.
There are several challenges regarding PHLF. First, there are no universal diagnostic criteria for reporting on PHLF in the literature making comparison of published articles difficult. Even with an increased usage of the PHLF criteria presented by the International Study Group of Liver Surgery (ISGLS) in 20113, numerous variations still occur in recent publications. Second, accurate preoperative methods for assessing whether the remnant liver after surgery will be sufficient to sustain function in the postoperative regenerative interval are lacking. Third, although liver regeneration has been studied extensively (mostly in animal models) and many pathways have been identified4,5, there is still no clear way to transfer this knowledge into clinically useful methods. Fourth, once PHLF occurs, we have no effective means to treat this condition despite many different attempts to support the regenerating remnant liver. So, presently the best way to treat PHLF is to avoid it from occurring.
A literature review was conducted to identify relevant articles for each topic of PHLF covered. The literature search was performed using Medical Subject Heading terms on PubMed for articles on PHLF in English until May 2022. A formal systematic literature search according to the PRISMA guidelines6 was not undertaken.
The aim of this review is to present the current body of evidence regarding several aspects of PHLF, provide insight into the pathophysiology of this condition, which measures can be undertaken before surgery to prevent PHLF from occurring and how to handle the patient if this feared complication still occurs.
Definitions and epidemiology
Definitions and prediction
Since the beginning of the 21st century, a lot of scientific effort has been undertaken to describe, classify, and predict PHLF. In the 1960s, there was a remarkable amount of knowledge about the physiological effects of major hepatic resections on liver function7; however, since that time, liver dysfunction has been described and measured in numerous ways, making it difficult to compare scientific reports in this field. In 2005, Balzan et al. published an article in which they were the first to systematically describe clinically relevant liver failure. Based on blood samples (bilirubin and prothrombin time) on postoperative day five, they could predict 60-day fatalities8. Another definition was proposed by Mullen et al. in 2010, focusing only on peak bilirubin in the postoperative course9. The major weakness of these two criteria was their binarity, only comparing PHLF to non-PHLF. To overcome this, the ISGLS developed a new definition based on an expert consensus meeting, providing criteria containing three different grades of PHLF (grade A–C)3. The grading is based on bilirubin and prothrombin time on or after postoperative day five and changes in the clinical course of patients undergoing hepatectomy. Presently, the ISGLS PHLF criteria are the most frequently used in literature to define PHLF. Several aspects of the ISGLS criteria have been discussed, such as the potentially lacking clinical relevance of grade A10,11 and that the criteria do not contain a distinction between primary and secondary liver failure1. A major problem of the definitions and predictive models mentioned above is their time point of applicability. On or after postoperative day five, there are limited possibilities left to substantially influence and potentially treat postoperative liver dysfunction, given the immediate onset of liver regeneration after hepatectomy13. Recently, perioperative lactate dynamics were found to be suitable for early recognition of PHLF and prediction of both rate and fatalities14; however, these attempts have not resulted in new and widely used definitions. Thus, to allow comparability of reported results, many suggest using the ISGLS criteria until a new definition, based on a broad international agreement, is available. New definitions should, however, address an urgent need to develop predictive models and definitions that can be applied in the first 48 h after surgery, to guide possible treatment decisions for these patients. The most used criteria of PHLF are presented in Table 1.
Table 1.
Summary of the most important definitions of posthepatectomy liver failure
| Definition (original publication) |
Description | Predictive value (original publication) |
Study population | Validation studies |
|---|---|---|---|---|
| ISGLS criteria (Rahbari et al.3) | Severity grading (PHLF grade A, B and C) based on clinical and laboratory parameters on or after postoperative day 5 | Grade A–C: perioperative (30-day) fatalities OR 13.80; 95% c.i. 4.27–44.61; 99% sensitivity and 91% specificity for detection of fatalities | Definition based on literature review and consensus of ISGLS members. Original publication refers to data from single-centre experience, 835 patients, year 2002–2010, 9% cirrhosis | Calthorpe et al.10 Sultana et al.16 Skrzypczyk et al.219 Rahbari et al.15 |
| 50:50, Balzan criteria (Balzan et al.8) |
Bilirubin and PT on postoperative day 5 | If bilirubin >50 μmol/l and PT <50% on postoperative day 5: Relative risk of death 66 (95% c.i. 30,147) 59% 60-day fatalities |
Single-centre, 775 patients, years 1998–2002, 12% cirrhosis | Calthorpe et al.10 Sultana et al.16 Skrzypczyk et al.219 |
| Mullen, peak bilirubin criteria (Mullen et al.9) |
Postoperative peak serum bilirubin concentration more than 7 mg/dl | Prediction of liver-related death: sensitivity 93%; specificity 94% 90-day mortality (OR 10.8), 90-day liver-related fatalities (OR 250) |
Three centres, 1059 non-cirrhotic patients, years 1995–2005 | Calthorpe et al.10 Skrzypczyk et al.219 Sultana et al.16 |
ISGLS, International Study Group of Liver Surgery; PT, prothrombin time.
Epidemiology
The incidence of PHLF is highly dependent on which definition is used and the reported cohort of patients (for example demographic data, diagnosis, and extent of resection)15. Using the ISGLS criteria, the incidence of PHLF in recent publications ranges between 9 per cent16 and about 20 per cent in western cohorts at tertiary centres14; however, in population-based studies, a significant difference in 90-day fatalities following hepatectomy has been reported when comparing low- and high-volume centres1,17. As PHLF has been found to be the single most important cause for 90-day fatalities2 and research regarding PHLF mostly originates from high-volume centres, a different incidence of PHLF in other settings could be possible. For example, in a recent study facilitating data from the National Surgical Quality Improvement Program database in the USA on both minor and major hepatectomies, the incidence of all ISGLS grades of PHLF was under 5 per cent18.
Several approaches have been undertaken to risk stratify patients before surgery. One PHLF risk score found that simple blood tests and extent of surgery could predict PHLF19. The combination of the aspartate aminotransferase/platelet ratio index (APRI) and albumin–bilirubin grade (ALBI) score, demonstrated good preoperative risk assessment of postoperative outcome, both in eastern hepatocellular cancer cohorts20, as well as in a population-based western setting, including PHLF (ISGLS grade C) and 30-day fatalities18. Another interesting approach has been undertaken in a French multicentre study, that developed a risk calculator for PHLF in patients with cirrhosis, considering both pre-, peri-, and postoperative variables21. The study tried to reflect the fact that the incidence of PHLF is not only influenced by preoperative factors, but also by peri- and postoperative events. Finally, there are some promising circulating factors in blood that have been assessed as preoperative predictors for PHLF22,23, but they will require further validation before their clinical use can be established.
It is presently unclear as to what extent these tools have been introduced in general clinical practice and whether some of these predictive methods are widely used for preoperative patient selection. Thus, it still is difficult to accurately predict the incidence and individual risk of patients to develop PHLF before surgery24; however, preoperative prediction might help to guide preventive measures and even treatments before hepatectomy in the future.
Aetiology
Basic science, loss of function, and liver regeneration
Modern liver surgery is only made possible by the liver’s unique ability to regenerate. While most patients recover rapidly after liver resection, some develop PHLF. In this context, it is important to note that the liver is challenged on multiple levels after hepatic resection. When there is significant liver volume loss, resulting in a significant reduction of available hepatocytes to maintain liver metabolic function (excretory and synthetic), regenerative activity also demands significant energy, ultimately potentially resulting in an ‘energy crisis’. Particularly, mitotic activity appears with a trade-off in functional activity25. With ongoing liver regeneration, energy levels of the liver slowly recover and allow the return of metabolic function. The higher hepatic tissue loss, the higher the mitotic rate26. This reduction of metabolic function affects multiple mechanisms relevant for physiological homeostasis. In this context, it is important to note that postoperative volumetry can be affected by oedema and therefore correlates poorly with postoperative liver dysfunction and functional liver recovery27.
An increase in portal venous pressure has been postulated as a critical initiator of postoperative liver regeneration28. Increased portal inflow, in comparison with liver volume, produces vascular shear stress and increased intrahepatic vascular resistance. This shear stress acts mainly on liver sinusoidal endothelial cells, as it changes levels of sinusoidal perfusion and leads to the release of nitric oxide and other hepatotrophic factors. Nitric oxide primes hepatocytes for proliferation by inhibiting S-adenosyl methionine, which in turn leads to upregulation of cyclins D1 and D229,30. Accumulating evidence suggests that after liver resection, an overwhelming increase in portal pressure results in deleterious effects on liver regeneration31. Some authors have even challenged the concept of the ‘small-for-size’ syndrome and argued that it is rather a ‘small-for-flow’ process. After transplantation, portal hyperperfusion and sinusoidal congestion are critically involved in hepatic failure32. The negative effects of an increase in portal venous pressure are further aggravated by the activation of the hepatic arterial buffer response, with a reduction of hepatic arterial perfusion and concomitant parenchymal ischaemia33. In line with this hypothesis, portal venous pressure increase after hepatic resection has recently been documented to correlate with hepatic dysfunction34. It is important to note that exploratory evidence has suggested that preoperative portal vein embolization (PVE) might alleviate sudden postresectional increase of portal venous pressure and might have an additional benefit if major resection is planned34. Further, changes in hepatic blood flow during liver resection lead to postoperative increased intrahepatic vascular resistance and in turn, to endogenous vasopressor release. An ‘acute hepatorenal-like syndrome’ because of arterial vasoconstriction can induce acute kidney injury35. Around 15 per cent of patients subjected to hepatic resections suffer from acute kidney injury, and this complication is associated with a mortality rate of up to 23 per cent. Through the haemodynamic and haemostatic changes explained above, development of acute kidney injury and hepatorenal syndrome is promoted by PHLF and often ends in multiple organ failure and sepsis.
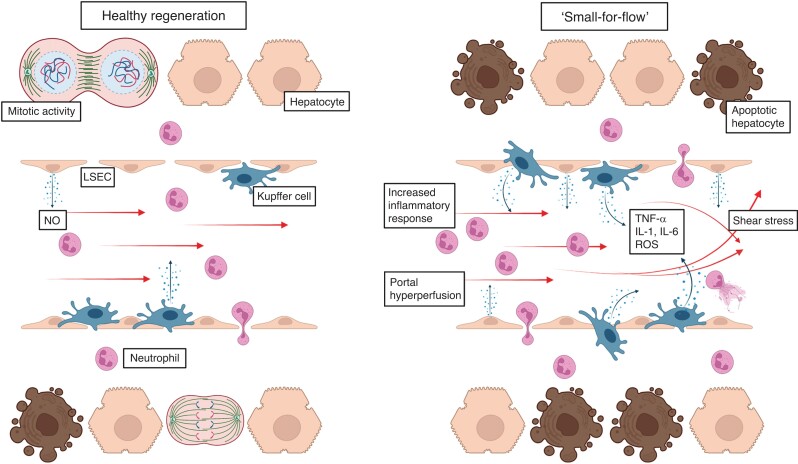
Initiation of liver regeneration occurs by a combination of several key signalling pathways, including mitogenic growth factors as well as multiple non-mitogenic cytokines. The growth factor receptors of hepatocyte growth factor and epidermal growth factor are key mitogenic receptors for both hepatocytes and progenitor cells36. The early phase of liver regeneration is initiated mainly by cytokines. An increase in tumour necrosis factor (TNF)-α activates transcription factors nuclear factor (NF)-κB in Kupffer cells and STAT-3 in hepatocytes37. Activated Kupffer cells in turn produce interleukin (IL)-6. IL-6 activates hepatocytes by binding to its receptors initiating proliferation38. Downstream activation of IL-6 and TNF-α is also amplified through an increased bacterial translocation into the liver. Bacterial endotoxins and blood-derived enteric microorganisms bind to Toll-like receptors in the liver, which also lead to the expression of these cytokines; however, while endotoxins like lipopolysaccharide induce cytokine expression, increased bacterial load in the liver, mainly because of hepatic inflow occlusion and reduced clearance of toxins, inhibits Kupffer cell function39,40. Disturbance of Kupffer cell function can lead to upregulated apoptosis and irreversible necrosis. Overshooting cytokines show a detrimental effect on liver regeneration41. An increased inflammatory response can induce systemic inflammatory response syndrome and ultimately end in PHLF and multiorgan failure42. An overwhelming immune response may be triggered through excessive bleeding or hepatic in- or outflow exclusion, which can induce hepatic ischaemia–reperfusion injury. Hepatic ischaemia and reperfusion lead to activation of the liver’s innate immune system. NF-κB, IL-6, TNF-α, reactive oxygen species, and chemokines are produced by Kupffer and endothelial cells. Although this is intended to facilitate liver regeneration, a disproportionate immune response can aggravate liver injury43. An important regulator of surgery-associated inflammatory signals is Kupffer cell plasticity. During the regenerative process, Kupffer cells switch from the pro-inflammatory M1 to the anti-inflammatory, pro-regenerative M2 phenotype. Inhibited phenotype change in Kupffer cells has been shown to negatively impact postoperative liver regeneration and promote occurrence of PHLF44. See Fig. 1 for a schematic presentation of healthy and dysfunctional liver regeneration.
Fig. 1.
Schematic overview of normal and dysfunctional liver regeneration
Left side shows functional liver regeneration. Major hepatectomy induces drastic changes in the haemodynamic environment of the liver. An increase in shear stress leads to activation of liver sinusoidal endothelial cells, which in turn release nitric oxide (NO). NO together with cytokines released from Kupffer cells, such as tumour necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 promote liver regeneration. Right side shows dysfunctional liver regeneration. An overwhelming increase in portal pressure can cause ‘small-for-flow‘ syndrome. Excessive shear stress induces an overshooting inflammatory response, followed by neutrophil recruitment into the liver. This causes inhibition of liver regeneration, parenchymal necrosis, and hepatocyte apoptosis. LSEC, liver sinusoidal endothelial cells; ROS, reactive oxygen species.
From bench to bedside
Up to now, to study the mechanisms of liver regeneration, animal models have been paramount. The 70 per cent partial hepatectomy model in rodents has given important insights into the physiological processes needed for postresection liver regeneration. A 90 per cent partial hepatectomy seems to approximate the human situation of a severe PHLF and mimics the ‘small-for-flow’ or ‘small-for-size’ syndrome45. As in humans, extensive resection leads to increased vascular stress and inhibited liver regeneration, due to sinusoidal endothelial cell damage and overshooting inflammation46. The use of mouse models has greatly increased the understanding of the role of various genes in human liver regeneration. Through different transgenic mouse strains, overexpression or depletion of target genes and the associated effect on liver regeneration after partial hepatectomy can be assessed47. While animal models have been shown to be very informative, there is still a gap in translation of these data into human relevance.
Clinical risk factors
Parameters associated with PHLF can be categorized into patient-, liver-, or surgery-associated factors. A summary of these factors can be found in Table 2.
Table 2.
Risk factors for posthepatectomy liver failure
| Patient-associated | |
| Sex | Risk double in males, especially males with HCC |
| Female hormones show proliferative effect in animal models, inhibiting effect of testosterone on immune system | |
| NAFLD, lower postoperative risk than other chronic liver diseases, higher incidence in postmenopausal women | |
| Age | Still unclear, possible changes in bile flow and acute-phase protein production |
| Age-related sinusoidal pseudocapillarization, rescue in animal models through serotonin agonist injection | |
| Sepsis | Bacterial endotoxins decrease cytokine production needed for liver regeneration |
| Kupffer cell and hepatocyte function in liver regeneration inhibited | |
| Metabolism | Insulin induces expression of IGF and HGF |
| High BMI and malnutrition associated with PHLF | |
| Other | Serum bilirubin, low platelets, insufficient renal function, cardiopulmonary disease, associated with PHLF |
| Liver-associated | |
| Steatosis | Leads to changes in the hepatic microenvironment and higher risk for ischaemia–reperfusion injury |
| Neoadjuvant chemotherapy | Chemotherapy-associated liver injury and steatohepatitis are known complications after neoadjuvant chemotherapy |
| Fibrosis grade | Functional liver tissue reserve is reduced, patients often present with several comorbidities |
| Cholestasis | Jaundice increases morbidity after surgery; in animal models, bile duct ligation leads to reduced growth factor expression |
| Portal hypertension | High preoperative portal pressure in cirrhosis associated with increased risk of PHLF |
| Surgery-associated | |
| Future liver remnant | ‘Small-for-flow’ syndrome negatively impacts hepatic haemodynamics |
| Increase in portal pressure leads to altered hepatic microcirculation and hepatocyte damage | |
| Blood loss | Leads to intravascular fluid shifts, introduction of bacterial endotoxins into the hepatic microenvironment |
| Increased risk of sepsis, coagulopathy and PHLF | |
| Surgical technique | Vascular occlusion can cause ischaemia–reperfusion injury and in increases PHLF risk |
| Long Pringle manoeuvre leads to increased oxidative stress and overshooting inflammatory response | |
| Extensive vascular resection can cause PHLF | |
HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease; IGF, insulin-like growth factor; HGF, hepatocyte growth factor; PHLF, posthepatectomy liver failure.
Patient-associated
Sex: the likelihood to develop PHLF is almost double in men and the risk of postoperative complications is also generally higher9. A possible explanation may be inhibition of immunocompetence through testosterone levels48. In a rat model, 17β-oestradiol injection led to accelerated liver regeneration49. In a study examining 13,401 patients, the risk for PHLF associated with men was especially pronounced in patients with hepatocellular carcinoma50. A possible explanation could be that the incidence of non-alcoholic fatty liver disease in postmenopausal women is higher than in men of the same age. In comparison with other chronic liver diseases such as viral hepatitis or alcoholic steatohepatitis, non-alcoholic fatty liver disease is associated with a lower postoperative risk50.
Age: it is still not fully understood how advanced age negatively impacts liver regeneration. With advanced age, bile flow and lower production of acute-phase proteins change and could influence liver regeneration51. Another explanation may be that age-related pseudocapillarization in the liver, leading to loss of fenestration in the sinusoidal space, inhibits liver regeneration. In a mouse model, liver regeneration could be rescued by injection of a serotonin receptor agonist, with the hypothesis that serotonin-mediated vascular endothelial growth factor release relaxes the sinusoidal lining52.
Sepsis: bacterial endotoxins interact with Kupffer cells and hepatocytes, inhibiting cytokine production needed in the early phase of liver regeneration53.
Metabolism: insulin is an important inducer of growth factors such as insulin-like growth factor and hepatocyte growth factor54. Animal models have shown that insulin depletion inhibits liver regeneration55. BMI and malnutrition also show an association with PHLF56,57.
Other: preoperative reduced renal function and cardiopulmonary disease also show a correlation with PHLF58, presumably as a reflection of the overall physical condition of the patient.
Liver-associated
Steatosis: hepatic steatosis leads to haemodynamic changes in liver sinusoids leading to an increased risk for ischaemia–reperfusion injury and raises the risk of postoperative complications59.
Neoadjuvant chemotherapy: neoadjuvant regimens containing irinotecan or oxaliplatin can cause chemotherapy-associated liver injury and have been shown to have an adverse effect on liver physiology and regeneration. Irinotecan has been associated with steatosis as well as steatohepatitis and patients receiving oxaliplatin-containing regimens are more likely to develop sinusoidal obstruction syndrome and biliary complications60,61.
Fibrosis grade: the presence of high-grade fibrosis or cirrhosis has a detrimental effect on patient outcome. The risk of fatalities rises with the extent of fibrosis62. Patients suffering from high-grade fibrosis or cirrhosis often also present with a plethora of co-morbidities such as portal hypertension, jaundice, or coagulopathy, which all increase PHLF risk63. Because of the advanced stage of chronic liver disease, functional liver tissue and liver reserve is reduced64 resulting in significantly reduced postoperative liver regeneration65.
Cholestasis: jaundice can significantly increase the risk of morbidity after surgery66. For example, animal models show a decrease in growth factor expression after bile duct ligation, which negatively impacts liver regeneration67. In addition, external bile duct drainage might lead to loss of bile salts and influence fibroblast growth factor 19, which could in turn hamper postoperative liver regeneration68,69.
Portal hypertension: patients with cirrhosis and portal hypertension have an increased risk of developing PHLF compared with patients with normal portal pressure70. Several attempts to predict and prevent PHLF in these patients have been proposed, such as the use of digital twins71. More commonly, invasive measurement of the hepatic vein pressure gradient is applied before surgery to predict PHLF72.
Surgery-associated
Future liver remnant (FLR): as explained above, ‘small-for-flow’ syndrome is responsible for drastic haemodynamic changes in the liver. Mainly induced by an intraoperative increase in portal pressure, microcirculation in the liver is altered, and hepatocyte damage occurs73,74.
Blood loss: excessive intraoperative blood loss leads to intravascular fluid shifts. This can lead to the introduction of bacteria and bacterial endotoxins to the hepatic microenvironment, increasing the risk of sepsis, coagulopathy, and PHLF. Intraoperative blood loss is directly associated with postoperative morbidity75.
Surgical technique: intermittent inflow occlusion or total vascular occlusion are surgical strategies that cause ischaemia–reperfusion injury during hepatic resection, which might increase the risk for PHLF76. Mechanistically, during vascular occlusion, Kupffer cells are activated and release pro-inflammatory cytokines such as TNF-α and IL-1 and reactive oxygen species. Lengthy vascular occlusion time leads to increased oxidant stress and immune response after reperfusion. Overshooting inflammatory response and oxidative stress can injure the liver and inhibit liver regeneration. Further, extensive vascular resection and reconstruction of the inferior vena cava has been shown to negatively impact postoperative outcome and cause PHLF. Extensive resection in the portal area and the hepatoduodenal ligament is also associated with PHLF77. Frequently used surgical devices in liver resection include the cavitron ultrasonic surgical aspirator and surgical staplers. Studies comparing the two did not show significant differences in postoperative morbidity or blood loss78. The stapler technique was, however, shown to be significantly faster than the cavitron ultrasonic surgical aspirator and was associated with lower levels of inflammatory cytokines, possibly due to shorter time under anaesthesia. This study, however, suffered from small a sample size, and a statement about specific mechanisms cannot be made79. Furthermore, selecting a laparoscopic approach when operating on cirrhotic patients with hepatocellular carcinoma seems to reduce the risk for PHLF compared with open resection80,81. In addition, laparoscopic resection of hepatocellular carcinoma in cirrhotic patients with portal hypertension and even Child–Pugh grade B seems feasible82,83, although high-grade evidence is missing.
Prevention
FLR volume and formulas
The risk of PHLF after liver resection is related to the size of the remnant liver; the smaller the remnant liver, the higher the odds of PHLF84–86. Preoperative estimation of liver volume based on three-dimensional reconstructions of contrast-enhanced CT images has been shown to correlate with actual liver weight and is the standard approach to estimate the risk of liver failure after resection84,87. FLR volume is usually expressed as the proportion of liver volume that remains after resection relative to the total liver volume excluding tumour volume and is expressed as a percentage as measured on CT images88. Alternatively, the measured FLR volume can be related to a calculated total estimated liver volume value using body composition parameters, resulting in a standardized FLR volume. The calculation of total estimated liver volume avoids the segmentation of liver tumours, which can be laborsome88–90. Similarly, FLR volume can be related to bodyweight to calculate the remnant liver volume to bodyweight ratio91. Despite small differences in predictive values across cohorts, all these calculations aim to guide clinicians towards safe liver resection. Table 3 describe the most common methods to calculate FLR volume in relation to the total liver volume or other body composition parameters.
Table 3.
Most common methods to assess future liver remnant size
| Formula | Method to calculate | Strengths | Limitations |
|---|---|---|---|
| FLR/TLV | (FLR ml/TLV ml)×100 | Accurate | Complicated, time-consuming |
| sFLR | (FLR ml/TELV ml (794–1267×BSA)) ×100 | Easy to perform, widely used currently | Potentially inaccurate for large tumours |
| FLR/BW-ratio | FLR in ml/BW in kg | Easy to perform | Rarely used |
FLR; future liver remnant; TLV, total liver volume; sFLR, standardized FLR; TELV, total estimated liver volume; BSA, body surface area; BW, bodyweight.
Numerous analyses have tried to establish a safe FLR volume cut-off above which PHLF can be avoided. For patients with otherwise healthy liver parenchyma, these cut-offs range from 20 to 30 per cent92,93. Most studies report low rates of liver failure (0–6 per cent) when the FLR volume is above the cut-off, and high rates (20–90 per cent) when the FLR volume is smaller92,94.
For patients with parenchymal liver disease, a similar size liver remnant is compromised in function; and consequently, the risk of PHLF is increased. When liver resection is performed in patients that suffer or have suffered from cholestasis or cirrhosis, the risk of PHLF increases95,96. FLR volume is a predictor of PHLF in both cirrhotic and cholestatic patients who undergo liver resection21,96. In these patients, a FLR volume of at least 40 per cent is suggested, and some studies recommend even 50 per cent in case of established cirrhosis21,84,85,95–98; however, the evidence to substantiate these cut-off levels is limited. The increasing risk of PHLF with a decreasing FLR size is evident. Most studies have tried to establish a cut-off value to select patients for liver resection; yet, the risk of PHLF in a patient with an FLR 1 per cent below any binary cut-off is likely not very different from a patient with a FLR 1 per cent above the same cut-off. Furthermore, the onset of PHLF is multifactorial and many risk factors have been identified, many of which are specific to disease subgroups99.
FLR volume is perhaps the most important and readily available preoperative parameter to estimate the risk for liver failure, but risk assessment using multiple factors is likely to be essential to truly stratify patients at risk for PHLF.
Methods for preoperative liver function assessment
FLR volume may provide an estimate of the remnant capacity for function and regeneration100; however, this only works under the assumption that the total liver function is intact and that its distribution is homogenous. Parenchymal damage and diminished liver function due to chemotherapy or underlying liver disease is not accounted for in normal volumetry and can only be assessed by preoperative liver function tests. In addition, after FLR augmentative procedures such as PVE distribution of function is no longer homogenous and volumetry in these situations is known to both under- and overestimate remnant function101. In these circumstances functional tests of the liver have the higher clinical value.
Although the liver is responsible for a wide range of functions, all methods for functional liver assessment focus on one specific part of liver function as a predictor of actual global liver function and of regenerative potential. For practical use, a test should be able to estimate the risk of PHLF, assess the need for FLR augmentative procedures, and be used to estimate the effect after such procedures. Based on the aforementioned practical issues with volumetry, two parts of functional tests are essential: estimation of total liver function and its distribution in the FLR. When both are combined, this leads to an estimate of the actual remnant function as a potential predictor for PHLF.
Laboratory tests and models such as ALBI and Model for End-stage Liver Disease provide screening tools for poor liver function but are only useful in the low range of total function102. The same can be said about the use of biopsies and elastography103. This makes these tests of limited value for providing information on the FLR function. Nevertheless, because of low costs and high availability, simple laboratory tests can be used as a screening tool for poor general liver function or limited regenerative potential due to an underlying liver condition.
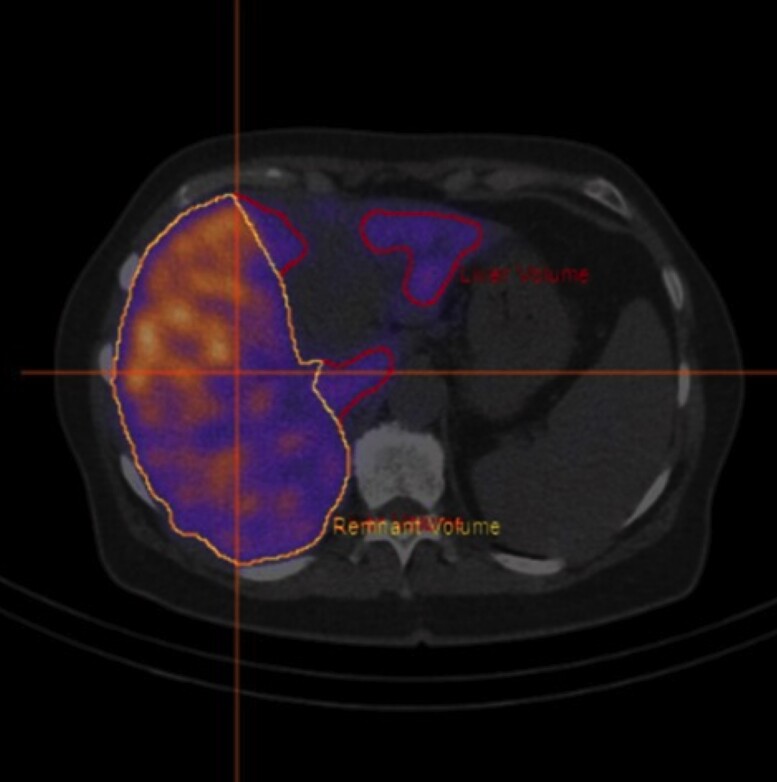
A wide range of tests and imaging methods is available to measure liver function before surgery (Table 4). There are no randomized trials or high-level evidence to support the use of any of these tests to predict PHLF better than volumetry; however, based on retrospective series and use in daily practice, there is consensus among experts that functional assessment of the remnant liver is essential for predicting PHLF in compromised and FLR-augmented livers (with European consensus guidelines in preparation). To understand the value of functional tests, it is important to explain the mechanisms behind commonly used methods, their respective advantages, and their drawbacks. First, there are tests that measure clearance or metabolism of a certain substance. The outcome is calculated in a multi-compartment model, and the outcome gives a measure of total function104,105. Examples are the indocyanine green (ICG) clearance and LIMAX (13C-methacetin breath) metabolic tests106,107. As the goal is to estimate FLR function, total liver function tests should always be accompanied by volumetric correlation, dividing the total function by the remnant volume share under the assumption of homogeneity of distribution within the liver. After FLR augmentative procedures, this method cannot measure the effect of the intervention on FLR function as it only gives information on total function and not regional functional distribution. A second group of tests combines clearance of an imageable agent, for instance 99mTc-Mebrofenin or 99mTc-GSA (DTPA-galactosyl serum albumin) and gadoxetic acid, with a three-dimensional structural scan (for hepatobiliary scintigraphy (HBS) and MRI)108–111. These methods provide accurate information about distribution of function, which is a clear advantage over total liver function tests; however, routine use is not widely adopted due to the complexity of acquisition, processing, and interpretation. The advantage of the MRI technique is that it could potentially serve as a one-stop-shop solution, providing structural diagnostic scans and functional information in one examination. A potential, but unusual, limitation is that some patients do not tolerate MRI scanning due to severe anxiety112. Basically, there are two ways that MRI can be used for clearance-based assessment. The most straightforward method is to measure relative enhancement of the liver compared with another organ, such as the spleen or muscle. In daily practice, this method seems to be able to predict PHLF quite well110; however besides the influence of flow and cardiac output, the technical nature of MRI scanning implies that measured values are, by definition, estimated and not absolute. The patient as well as the multitude of different types of MRI scanners and vendors combined with local conditions further complicate inter-patient and inter-centre comparability. The result is an uncalibrated value of enhancement, and thus a function measurement that cannot be compared between patients and no clear cut-offs for safe resections can be given. An alternative to relative methods is to measure the clearance over time using a dynamic scanning protocol. This provides a slope ‘K-value’, and therefore, an actual estimate of the speed of clearance and more closely resembles other clearance measurements (such as the ICG test or HBS); however, these protocols require longer acquisition times, are highly complex and difficult to implement110. Hepatobiliary scintigraphy is based on clearance of an isotope-labelled, liver-specific agent such as 99mTc-Mebrofenin113. Processing is similar to a dynamic MRI, however, acquisition is much less complex108. Interpretation is straightforward, and due to good comparability between centres, it has been validated more systematically, which has led to a practical clinical cut-off value114. When combined with single-photon emission CT, some anatomical mapping and FLR calculation can also be acquired115, although with clearly inferior quality compared with MRI. All described clearance methods may suffer from accumulation of the cleared agent in the bile ducts, and masking of this signal is sometimes needed to compensate for interference. This significantly adds to the complexity of the processing and interpretation108. Another drawback is that the clearance may be hampered in patients with cholestasis116. The transporter proteins are dysregulated, and the agent must compete with bilirubin for transport in and out from the hepatocyte resulting in a low measure of function. This does not mean that the measurement is impossible to use in jaundiced patients, it just clearly shows that these patients suffer from hepatic dysfunction. After biliary drainage and restoration to normal levels of bilirubin, the test can be repeated and should show a normalized function, more accurately resembling the actual postoperative remnant function. An alternative for 99mTc-mebrofenin is 99mTc-GSA. 99mTc-GSA is albumin-bound and liver specific and not dependent of bilirubin levels, making its application less complicated in jaundiced patients; however, GSA is not registered in most western countries, limiting its use. The information on regional distribution of function supplied by HBS and MRI can be very useful in jaundiced or insufficiently drained patients, showing dysfunctional or poorly drained segments that may require further interventions to improve remnant function. Fig. 2 shows a 99mTc-Mebrofenin scan with poor biliary drainage of the right posterior sector, remnant function was below the safe threshold for resection and improved sufficiently after additional biliary drainage.
Table 4.
Overview preoperative tests to estimate adequate remnant liver function
| Test | Agent used | FLR volume | TL function | FLR function | Distribution | FLR function after | Complexity | Validated for PHLF |
|---|---|---|---|---|---|---|---|---|
| Volumetry | Yes | No | No | No | – | – | ++ | |
| Laboratory scores | No | No* | No | No | – | – | + | |
| ICG test | Indocyanine green | No | Yes | No | No | – | + | ++ |
| LIMAX | 13C-methacetin | No | Yes | No | No | – | + | + |
| LIMAX+volumetry | 13C-methacetin | Yes | Yes | Yes | No | + | ++ | + |
| ICG+volumetry | Indocyanine green | Yes | Yes | Yes | No | + | ++ | ++ |
| HBS | 99mTc-Mebrofenin | Yes | Yes | Yes | Yes | ++ | ++ | ++ |
| RLE-MRI | Gadoxetic Acid | Yes | Limited† | Limited† | Yes | +† | + | + |
| DCE-MRI | Gadoxetic Acid | Yes | Yes | Yes | Yes | ++ | +++ | – |
ICG, Indocyanine green; HBS, hepatobiliary scintigraphy; RLE, relative liver enhancement; MRI, magnetic resonance imaging; DCE, dynamic contrast-enhanced; FLR, future liver remnant; TL, total liver; PHLF, posthepatectomy liver failure; +, low; ++, medium; +++, high. *Only sensitive in low liver function/cirrhosis. †Not absolute.
Fig. 2.
Regional distribution of liver function assessed with hepatobiliary scintigraphy
99mTc-mebrofinin scan showing poor drainage of the right posterior sector, as can be seen by the higher (yellow signal) compared with low (blue).
For practical use, volumetry combined with a general functional test as screening tool for a compromised liver might work well in daily practice; however, when augmentative procedures are used, the advantage of imaging of distribution of function is evident and more complex methods are required. Choice of functional modality depend largely on local availability and expertise.
Modulation of the FLR
When the remnant liver is deemed insufficient for safe liver resection, a preoperative procedure to increase the liver remnant volume, and hopefully the corresponding function, can be performed. Preoperative PVE is the most accepted approach. The embolization of the portal branches to the segments intended for resection results in a compensatory growth of the FLR. While there are many studies on PVE that focus on the hypertrophic response and outcome after surgery following PVE, true comparative analysis on the impact of PVE on liver failure are sparse117–119. In the only prospective trial to date, PVE was associated with fewer complications in patients with chronic liver disease, but not in those with normal liver parenchyma; however, the trial did not include an FLR cut-off for inclusion, and the mean FLR volume was 30 per cent before PVE which might indicate that study population was not at a high risk of liver failure. Indeed, there was no liver failure in patients with normal liver parenchyma, and only two patients with liver failure in the chronic liver disease group, one with and one without PVE120. In patients with biliary tumours who have a high risk of liver failure, a matched study showed that PVE was associated with substantial reductions in PHLF rates121. Indeed, a more liberal approach towards PVE in these patients probably results in better outcomes122,123. Most other studies are non-comparative studies that show that liver resection can be performed with acceptable outcomes in patients in whom resection was not feasible without PVE124–128. The ability of the FLR to grow after PVE has been identified as an important predictor of a good outcome after resection129. When limited FLR growth is observed after PVE, subsequent resection has been shown to be associated with poor outcomes and can be a reason not to proceed to surgery. While tumour progression is the main reason for patients not to proceed to surgery after PVE, the absence of hypertrophy in some patients has fuelled the search for more effective liver regenerative strategies.
Before the introduction of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), two-stage hepatectomies had been used for more than a decade130,131. ALPPS is an accelerated two-stage procedure, combining portal vein occlusion with (partial) parenchymal transection to induce a rapid growth of the FLR132. The rapid hypertrophy allows for an earlier final resection, thereby increasing the resection rate compared with that of PVE133,134. Yet, liver failure and mortality rates remain higher with ALPPS despite the rapid liver growth133,135. Interestingly, none of the parameters on liver growth are related to postoperative outcomes. Only baseline FLR size is correlated to the overall outcomes136. Therefore, it can be argued that to make ALPPS safer, PVE should be attempted first to minimize the risk of PHLF137,138; however, such an approach would probably result in a lower resection rate, but it can be questioned whether the resection rate is worth the high 90-day fatalities134,139, even if recent publications on the use of ALPPS in patients with colorectal liver metastases show improved safety compared with initial series140.
In the search for more and faster hypertrophy without the increased risk of liver failure and fatalities seen in ALPPS, a simultaneous PVE and hepatic vein embolization (HVE) has emerged as promising alternative141. This combination (PVE/HVE) means that PVE (generally right-sided) is combined with occlusion of the hepatic vein that drains the deportalized side of the liver. Although it is thought that PVE/HVE results in greater hypertrophy compared with PVE alone, comparative studies have still not shown a difference42–146. Resection rates and outcomes after resection following PVE/HVE were also similar to PVE alone147. PVE/HVE seems a safe alternative, and future studies should show whether this new technique provides a benefit over PVE alone. Radiological examples of PVE, ALPPS, and PVE/HVE are depicted in Fig. 3.
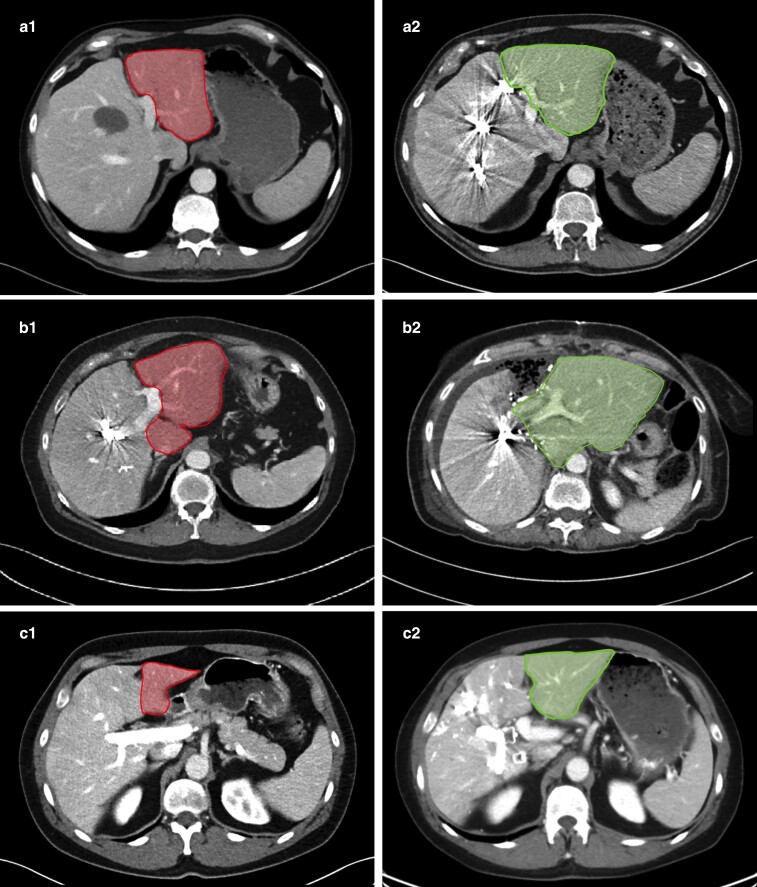
Fig. 3.
Methods to increase future liver remnant size
Contrast-enhanced CT of patients subject to different treatments. a PVE. b Rescue ALPPS after insufficient effect of PVE. c PVE/HVE. Before (1) and after (2) images are shown in each case. All patients with left lateral segment (plus/minus segment 1) as FLR marked with red before intervention and green at evaluating radiology. PVE, portal vein embolization; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; HVE, hepatic vein embolization; FLR, future liver remnant.
Intraoperative and postoperative techniques for prevention of PHLF
Pringle manoeuvre
The Pringle manoeuvre was initially described to temporarily occlude the inflow to the liver with a soft clamp to control bleeding from the liver injury in the setting of liver trauma148. Over the years, liver surgeons widely adopted the Pringle manoeuvre in liver resection surgery and demonstrated reduced intraoperative bleeding and operative time149,150; however, ischaemia followed by reperfusion of the liver has been argued to cause injury to the hepatocyte metabolism151, resulting in cytolysis. The ischaemia–reperfusion injury results in insufficient hepatic synthesis of acute-phase proteins and coagulation factors. The resulting changes in the inflammatory cascade increase the rates of posthepatectomy liver dysfunction and an impaired immune defence against bacterial infections152–154. Splanchnic vascular stasis due to the Pringle manoeuvre also damages enterocytes, loss of gut barrier, and contributes to endotoxaemia155. The increased bacterial translocation and endotoxins to the liver from portal circulation directly affect liver regeneration by impairing the initiator cytokines and causing direct damage to hepatocytes, resulting in cellular death. Ischaemic preconditioning has been proposed to be beneficial in animal models156, but its potential benefit remains to be demonstrated more clearly in humans57. Several randomized clinical trials investigated whether an intermittent Pringle manoeuvre would reduce some of the detrimental effects of a continuous Pringle manoeuvre; however, most of the studies failed to demonstrate clinically significant benefits of an intermittent over continuous Pringle manoeuvre158, while some studies continue to caution against the Pringle manoeuvre159,160. Fagenson et al.159, in a propensity-matched study, reported increased rates of PHLF and septic shock with the use of the Pringle manoeuvre for partial hepatectomies. Hemihepatic vascular inflow occlusion on the ipsilateral side of resection after isolating the vessels is reported to be better tolerated161. Ishizuka et al. reported shorter postoperative survival with longer Pringle manoeuvre time in patients undergoing resection for hepatocellular cancer162. Huang et al.163 compared a 25-min intermittent Pringle manoeuvre with a 15-min occlusion for resection of hepatocellular carcinoma. They reported a higher speed of liver transection (1.38 versus 1.23 cm2/min, P = 0.002) and lower blood loss during transection (109 versus 166 ml, P < 0.001) than the 15-min intermittent Pringle manoeuvre group. The authors of the present review do not believe that using the Pringle manoeuvre is mandatory for achieving good outcomes in liver resection, nor that it negatively influences outcomes when applied shortly. When the Pringle manoeuvre is used, the preference should be for judicious use of intermittent Pringle, preferably selective to the side of resection and for the shortest duration of parenchymal transection.
Intraoperative blood loss
Blood loss during liver surgery is determined by the complexity of liver resection, background liver parenchymal characteristics such as the presence of steatohepatitis or chemotherapy-induced liver injury, and the individual surgeon's experience. Intraoperative blood loss of more than 1200 ml and the need for perioperative blood transfusion are considered risk factors for PHLF164. The haemodynamic instability due to blood loss results in ischaemia–reperfusion injury. The need for blood transfusions results in potential immunosuppressive effects. With appropriate measures of low central venous pressure, judicious use of energy devices, and cavitron ultrasonic surgical aspirator, blood loss can be kept to a minimum.
Haemostasis and bile leaks to prevent postoperative sepsis
Sepsis is one of the common causes of death in patients with established PHLF. In addition, patients with liver failure are prone to develop sepsis, and sepsis due to any cause can also exacerbate PHLF12. The hypodynamic circulation and multiple organ failure associated with sepsis can result in hypoperfusion of the remnant liver, and it can further reduce the functional mass of Kupffer cells that play a pivotal role in the cytokine and IL regulation required for liver regeneration. Postoperative sepsis could be due to multi-site and multifactorial issues, of which intra-abdominal collections due to infected hematomas and biliary collections are some of the common causes. Meticulous haemostasis and attention to biliary stasis are essential in preventing postoperative collections following major liver resections. Several intraoperative measures such as transcystic air leak test165, portal re-occlusion166, and white test with lipid solution167 were described for localizing leaking biliary radicles on the transection surface. These tests can be used to identify and control the bile leak so the risk of postoperative biloma is reduced. As bile salt depletion could also affect liver regeneration negatively, large amount of externally drained bile (found and drained after surgery) should probably be returned to the patients enteric circulation68.
Flow modulation
It is increasingly realized that PHLF is not only a small remnant volume issue. The function of the FLR and the portal flow are also realized to be part of the pathophysiological process. The mechanics of hepatic inflow form the basis for applying modulatory flow principles and are implied in liver resections to reduce the risk of liver dysfunction. Portal flow modulation techniques, including portal flow diversion, splenic artery ligation, and splenectomy have long been applied in living-donor liver transplantation. Increased flow and pressure in the portal vein result in shear stress on sinusoidal endothelial cells that release nitric oxide, promoting liver regeneration. Ironically, excessive portal venous flow for the volume of the liver parenchyma leads to increased sinusoidal pressure, endothelial damage, and sinusoidal haemorrhage. High portal vein pressures also result in hepatic artery buffer response by reducing hepatic artery pressures leading to ischaemic biliary injury168. Regenerative response of the liver requires a balanced increase in the portal venous flow leading to regenerative stimulation but not up to the onset of hepatocytes injury. In addition, a reduced portal venous flow stimulates the hepatic arterial buffer response resulting in an increased hepatic tissue pO2 due to raised arterial flow and improved liver regeneration in animal studies169. Troisi et al.170 reported, and it is widely adopted, that a portal venous flow rate of more than 250 ml/min/100 g or a portal venous pressure of more than 20 mmHg is associated with poor outcomes. These detrimental effects get augmented when the liver volumes are less than optimal.
Splenectomy and splenic artery ligation
Splenic blood flow contributes to 25–30 per cent of the total portal flow. The percentage contribution of splenic flow to the remnant liver is much higher following major hepatectomy resulting in increased portal pressures. The role of splenectomy in flow modulation and preventing liver dysfunction is well described in living-donor liver transplantation; however, its role in extended hepatectomies is only described in animal studies. Splenectomy increases vascular compliance and hepatic serotonin levels, which improve hepatic perfusion through its vasodilatory effect. Serotonin exerts a protective effect by increasing microcirculation and accelerating liver regeneration by stimulating endothelial cells to release vascular endothelial growth factors171,172. Splenectomy enhances DNA synthesis and proliferation of cell nuclear antigens to facilitate liver regeneration in rats undergoing major hepatectomies. Risks of splenectomy include intraoperative bleeding, opportunistic postsplenectomy infection, and the need for long-term antibiotics. Splenic artery ligation is also an effective way to reduce the portal venous pressure and increase hepatic artery flow173. The arterial inflow from short gastric arteries will help preserve splenic parenchyma, although splenic infarction, splenic abscess, and pancreatitis have been described.
Pharmacological intervention of portal venous flow
Pharmacological modulation of portal venous flow using somatostatin analogues such as octreotide were explored as an alternative to splenectomy and splenic artery ligation. Somatostatin blocks the SSTR2 receptors on the endothelial cells of splanchnic vasculature resulting in vasoconstriction, reduced splanchnic flow, and decreased portal venous pressure. In addition, it suppresses hepatocellular proliferation but encourages a more regular and orderly regeneration. An intraoperative bolus dose of somatostatin bolus (250 μg) followed by a continuous 250 μg/h infusion for 5 days was used174. Animal studies have shown a marked reduction in portal venous flow and pressure and attenuation of liver injury after 80 per cent and 90 per cent hepatectomies with rapid and effective flow modulation175–177. In smaller clinical studies, when portal venous pressure is greater than 20 mmHg, somatostatin has immediately reduced the pressures by 2.5 mmHg174. Whether this translates to significant clinical benefit needs to be assessed in the larger ongoing clinical trials (such as SOMAPROTECT01; registration number: NCT02799212, http://www.clinicaltrials.gov). Another randomized clinical trial evaluated whether terlipressin could influence postoperative outcome after liver resection, without demonstrating a positive effect in the intervention arm178.
Intraoperative N-acetylcysteine administration
To limit oxidative cellular injury, N-acetylcysteine infusion has been used to clear (scavenging) the excess oxygen free radicles produced due to ischaemia–reperfusion injury179; however, clinical studies failed to demonstrate a clinical benefit of its use180,181. Timing of the administered treatment and limited sample size in these trials might have influenced the results negatively.
Intraoperative steroids
Glucocorticoids are potent anti-inflammatory drugs that modulate inflammatory and anti-inflammatory pathways. The role of steroids in altering the inflammatory pathways that can lead to systemic inflammatory response syndrome was evaluated in several randomized clinical trials182–185. Methylprednisolone 500 mg before induction or up to 90 min before surgery was used. These studies have demonstrated favourable postoperative changes in laboratory markers of systemic inflammation, including IL-6, IL-10, TNF-α, C reactive protein, liver function tests, and prothrombin time. In a meta-analysis by Richardson et al.186, preoperative steroids were associated with statistically significant reductions in serum bilirubin and IL-6 in the early postoperative interval. There was a trend towards a lower incidence of postoperative complications and prothrombin time, but it did not reach statistical significance.
Treatment
In general, current treatment recommendations for severe PHLF are based on treatment algorithms developed to support patients with acute liver failure due to other reasons than PHLF187. The most important goal is to support organ function, and thus, provide a chance for the failing liver the recover spontaneously. Symptomatic treatment for PHLF include all aspects of modern organ and patient support available at specialized intensive care units. This has been described extensively before and will not be repeated in this review31,188,189. In the following section specific aspects of PHLF treatment regarding medical treatment, extracorporeal liver support systems, and liver transplantation (LTx) will be discussed.
Medical/cell-derived treatment
Presently, there is not a single drug, nor a combination of different agents available to cure patients with PHLF; however over the past years, several treatment strategies have been evaluated. Some of them, such as aggressive treatment of infectious complications are established and uncontroversial, whereas others might be considered experimental.
Antibiotics
Appropriate steps must be taken to prevent postoperative sources of infection. Early mobilization, fast return to oral intake, and early removal of drains as part of an enhanced recovery programme showed a reduction in the risk of infectious complications190. Early recognition of sources of sepsis with appropriate radiological imaging, drainage of infected collections, and appropriate antibiotics are all important in controlling the infection that could induce or aggravate PHLF191. A recent meta-analysis found no benefit in postoperative prophylactic administration of antibiotics in patients following hepatectomy in terms of rate and fatalities192; however when PHLF is diagnosed, aggressive antimicrobial treatment is advised as infectious complications and sepsis are both common in PHLF, increasing the risk for adverse patient outcomes193.
Lactulose
Hepatic encephalopathy is a severe complication in patients with PHLF, mainly triggered by increased ammonia due to insufficient metabolism in the liver194. Treatment for patients with PHLF follows the same recommendations for treatment as patients with acute liver failure, and high-dose lactulose is recommended routinely94.
Stem cells
Even though stem cell therapy might not be considered a classical medical treatment, it probably represents one of the most promising treatment alternatives for patients with PHLF in the future. Over the past years numerous publications have been addressing different treatment aspects, mainly in liver diseases such as fibrosis195 and end-stage liver disease196. Results following mesenchymal stem cell transplantation in a porcine hepatectomy model have shown promising results197,198; however, this treatment presently has not reached clinical practice, and several limitations remain critical, such as target-organ infiltration and the number of administered cells199.
Extracorporeal liver support
Theoretically, extracorporeal liver support systems represent an appealing approach to bridge an impaired liver function in the immediate postoperative phase; however, these devices are mainly developed to support failing liver function in patients with acute or acute-on-chronic liver failure200. For these patients, it was hypothesized that the removal of not only water-soluble but also albumin-bound toxins would assist in detoxification and support global liver function until recovery. The first available device was the molecular adsorbent circulating system (MARS)201,202. MARS treatment has demonstrated improved liver detoxification and haemodynamics in patients with both acute and acute-on-chronic liver failure203,204; however, MARS treatment did not result in improved survival in two large randomized clinical trials, neither in acute-on-chronic205 nor in acute liver failure206. Other techniques, such as the single-pass albumin dialysis and plasma separation and filtration, have been developed207 and demonstrated comparable results to MARS in terms of their detoxification capacity200,208,209. In contrast, high-volume plasma exchange achieved improved transplant-free survival in patients with acute liver failure in a randomized clinical trial210. In the PHLF setting, however, only the MARS system has been systematically evaluated. In a prospective study, MARS was found to be safe and feasible to use in patients with PHLF early after hepatectomy211; however in a systematic review, it was not possible to provide evidence to support a routine use of MARS in patients with PHLF212. Other devices, such as bioartificial liver support systems, could potentially increase the benefit of extracorporeal liver support systems, adding additional hepatic functions like synthesis of proteins and coagulation factors on top of the detoxification. In 2004, a randomized clinical trial evaluating a porcine hepatocyte-based bioartificial liver support system in patients with acute liver failure could not demonstrate a significant survival benefit for the patients in the intervention arm213. Since then, different systems have been developed, mainly based on porcine derived hepatocytes214. Due to legal reasons in many countries, there is a need to use human-derived hepatocytes in bioartificial liver support systems because xenotransplantation is widely prohibited. Such systems are currently under evaluation in both animal and human studies but are not yet available for routine clinical use215.
Liver transplantation and PHLF
Liver transplantation is frequently stated to be the only definite treatment in patients with PHLF; however, the scientific evidence is low, and there are many obvious limitations. So far, three articles have described the experience with LTx in PHLF, two single-centre series216,217 and a recent multicentre experience218. All concluded that it is safe and feasible to offer LTx to patients with PHLF with good long-term outcomes comparable to LTx for established indications; however, there are many unanswered questions such as the optimal time point for LTx and how to justify this indication in light of donor organ shortages. Thus, LTx will probably remain a rescue treatment in expert centres for patients with benign histopathology or a diagnosis already accepted for LTx even without PHLF (for example hepatocellular cancer according to national guidelines). To provide access to LTx for patients with PHLF, it is necessary to have national strategies as well as established cooperation between liver transplantation units and hospitals performing hepatectomies.
Discussion and future perspectives
In this review, the evidence for the most important aspects on PHLF has been summarized. Evident to the reader is the absence of high-grade evidence for most aspects of PHLF research. Furthermore, the lack of accurate tools to predict PHLF and effective treatment adds to the clinical challenge of PHLF for hepatobiliary surgeons worldwide.
As seen in this review there are countless attempts to define PHLF. In the paper where the ISGLS PHLF criteria were presented for the first time, Rahbari et al. listed almost 50 different publications with its own corresponding definition of PHLF between 2003 and 20093. Since then, numerous additional articles presenting new definitions of PHLF have been published, many of them originating from single centres without validation. Whether this is a result of a disagreement with the ISGLS criteria or a real effort to improve the definition of PHLF is presently unclear. In 2017, Skrzypczyk et al. published an article comparing the ISGLS, Balzan 50:50 and peak bilirubin criteria with regard to their value in predicting severe outcomes related to PHLF219. This article was commented on by Harrison et al.220, discussing potential flaws of studies comparing binary predictive scoring systems, both from a statistical and clinical point of view. The following reply by Skrzypczyk et al.221, however, vividly demonstrate the lack of agreement on PHLF-related questions in the hepatobiliary surgical community. From a clinical point of view, it would be desirable to have a definition for every patient undergoing hepatectomy, with high accuracy and applicable as early as possible after surgery, to guide the surgeon in selecting early postoperative treatment strategies (that are currently lacking); however, the implementation of a new definition should be counselled by an organization such as ISGLS or International Hepato-Pancreato-Biliary Association and not by several individual single centres. So, the recommendation until then is to urge the hepatobiliary community to use the ISGLS criteria in publications on PHLF to allow comparison between studies.
Liver volume measurements are the cornerstone to estimate the risk before surgery of PHLF after liver resection. Despite the great number of studies, most studies focus on a binary volume cut-off, whereas the risk of PHLF gradually increases with a decreasing FLR size. Furthermore, data on very large cohorts remain limited, and future international collaborative prospective trials might be the way forward. Most research on liver regenerative procedures focusses on the extent and speed of liver growth and the resection rate. Yet, it remains to be established whether an increased resection rate also provides an oncological benefit or whether the test of time that prevents surgery in patients with unfavourable tumour biology is more important.
The development of an accurate and easily available liver function test with capacity to measure regional function would probably be the most important tool to prevent PHLF. This could assist the hepatobiliary surgeon not only in avoiding resection in patients with limited FLR function (or submit them for FLR-augmenting procedures) but also potentially reveal sufficient FLR function in patients that today are deemed unresectable with current methods.
Given the relevant differences between mice and men, identification and characterization of pathophysiological processes dysregulated in patients developing PHLF is key to identify potential new treatment targets. Clinically applicable, integrative models, including preoperative modifiable and non-modifiable risk factors, might improve preoperative risk assessment in patients undergoing hepatic resection.
Contributor Information
Ernesto Sparrelid, Department of Clinical Science, Intervention and Technology, Division of Surgery, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Pim B Olthof, Department of Surgery, Erasmus MC, Rotterdam, The Netherlands; Department of Surgery, Amsterdam UMC, Amsterdam, The Netherlands.
Bobby V M Dasari, Department of HPB Surgery and Liver Transplantation, Queen Elizabeth Hospital, Birmingham, UK; University of Birmingham, Birmingham, UK.
Joris I Erdmann, Department of Surgery, Amsterdam UMC, Amsterdam, The Netherlands.
Jonas Santol, Department of Surgery, HPB Center, Viennese Health Network, Clinic Favoriten and Sigmund Freud Private University, Vienna, Austria; Department of Vascular Biology and Thrombosis Research, Centre of Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria.
Patrick Starlinger, Division of General Surgery, Department of Surgery, Medical University of Vienna, General Hospital of Vienna, Vienna, Austria; Department of Surgery, Division of Hepatobiliary and Pancreas Surgery, Mayo Clinic, Rochester, New York, USA.
Stefan Gilg, Department of Clinical Science, Intervention and Technology, Division of Surgery, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Data availability
This is narrative review and no data have been generated by the authors.
References
- 1. Gilg S, Sparrelid E, Isaksson B, Lundell L, Nowak G, Stromberg C. Mortality-related risk factors and long-term survival after 4460 liver resections in Sweden-a population-based study. Langenbecks Arch Surg 2017;402:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilg S, Sandstrom P, Rizell M, Lindell G, Ardnor B, Stromberg Cet al. . The impact of post-hepatectomy liver failure on mortality: a population-based study. Scand J Gastroenterol 2018;53:1335–1339 [DOI] [PubMed] [Google Scholar]
- 3. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam Ret al. . Posthepatectomy liver failure: a definition and grading by the International Study Group Of Liver Surgery (ISGLS). Surgery 2011;149:713–724 [DOI] [PubMed] [Google Scholar]
- 4. Michalopoulos GK. Liver regeneration. J Cell Physiol 2007;213:286–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol 2021;18:40–55 [DOI] [PubMed] [Google Scholar]
- 6. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stone HH, Long WD, SmithRB, 3rdHaynes CD. Physiologic considerations in major hepatic resections. Am J Surg. 1969;117:78–84 [DOI] [PubMed] [Google Scholar]
- 8. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse Det al. . The “50–50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824–828; discussion 8–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam Set al. . Hepatic insufficiency and mortality in 1059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854–862; discussion 62–4 [DOI] [PubMed] [Google Scholar]
- 10. Calthorpe L, Rashidian N, Benedetti Cacciaguerra A, Conroy PC, Hibi T, Hilal MAet al. . Using the comprehensive complication index to rethink the ISGLS criteria for post-hepatectomy liver failure in an international cohort of major hepatectomies. Ann Surg 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumgartner R, Gilg S, Bjornsson B, Hasselgren K, Ghorbani P, Sauter Cet al. . Impact of post-hepatectomy liver failure on morbidity and short- and long-term survival after major hepatectomy. BJS Open 2022;6:zrac097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Keulen AM, Buettner S, Besselink MG, Busch OR, van Gulik TM JNMIJet al. . Primary and secondary liver failure after major liver resection for perihilar cholangiocarcinoma. Surgery 2021;170:1024–1030 [DOI] [PubMed] [Google Scholar]
- 13. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niederwieser T, Braunwarth E, Dasari BVM, Pufal K, Szatmary P, Hackl Het al. . Early postoperative arterial lactate concentrations to stratify risk of post-hepatectomy liver failure. Br J Surg 2021;108:1360–1370 [DOI] [PubMed] [Google Scholar]
- 15. Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Buchler MWet al. . The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol 2011;18:3640–3649 [DOI] [PubMed] [Google Scholar]
- 16. Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JNet al. . Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford) 2018;20:462–469. [DOI] [PubMed] [Google Scholar]
- 17. Filmann N, Walter D, Schadde E, Bruns C, Keck T, Lang Het al. . Mortality after liver surgery in Germany. Br J Surg 2019;106:1523–1529 [DOI] [PubMed] [Google Scholar]
- 18. Starlinger P, Ubl DS, Hackl H, Starlinger J, Nagorney DM, Smoot RLet al. . Combined APRI/ALBI score to predict mortality after hepatic resection. BJS Open 2021;5:zraa043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dasari BVM, Hodson J, Roberts KJ, Sutcliffe RP, Marudanayagam R, Mirza DFet al. . Developing and validating a pre-operative risk score to predict post-hepatectomy liver failure. HPB (Oxford) 2019;21:539–546 [DOI] [PubMed] [Google Scholar]
- 20. Shi JY, Sun LY, Quan B, Xing H, Li C, Liang Let al. . A novel online calculator based on noninvasive markers (ALBI and APRI) for predicting post-hepatectomy liver failure in patients with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2021;45:101534. [DOI] [PubMed] [Google Scholar]
- 21. Prodeau M, Drumez E, Duhamel A, Vibert E, Farges O, Lassailly Get al. . An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J Hepatol 2019;71:920–929 [DOI] [PubMed] [Google Scholar]
- 22. Starlinger P, Hackl H, Pereyra D, Skalicky S, Geiger E, Finsterbusch Met al. . Predicting postoperative liver dysfunction based on blood-derived MicroRNA signatures. Hepatology 2019;69:2636–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Starlinger P, Pereyra D, Haegele S, Braeuer P, Oehlberger L, Primavesi Fet al. . Perioperative von Willebrand factor dynamics are associated with liver regeneration and predict outcome after liver resection. Hepatology 2018;67:1516–1530 [DOI] [PubMed] [Google Scholar]
- 24. Truant S, El Amrani M, Skrzypczyk C, Boleslawski E, Sergent G, Hebbar Met al. . Factors associated with fatal liver failure after extended hepatectomy. HPB (Oxford) 2017;19:682–687 [DOI] [PubMed] [Google Scholar]
- 25. Yokoyama Y, Nishio H, Ebata T, Igami T, Sugawara G, Nagino M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg 2010;97:1260–1268 [DOI] [PubMed] [Google Scholar]
- 26. Kobayashi T, Imamura H, Aoki T, Sugawara Y, Kokudo N, Makuuchi M. Morphological regeneration and hepatic functional mass after right hemihepatectomy. Dig Surg 2006;23:44–50 [DOI] [PubMed] [Google Scholar]
- 27. Kwong AJ, Goel A, Mannalithara A, Kim WR. Improved posttransplant mortality after share 35 for liver transplantation. Hepatology 2018;67:273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg 2007;31:367–374 [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001;294:559–563 [DOI] [PubMed] [Google Scholar]
- 30. Kin Y, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Nagino Met al. . Doppler analysis of hepatic blood flow predicts liver dysfunction after major hepatectomy. World J Surg 1994;18:143–149 [DOI] [PubMed] [Google Scholar]
- 31. van Mierlo KM, Schaap FG, Dejong CH, Olde Damink SW. Liver resection for cancer: new developments in prediction, prevention and management of postresectional liver failure. J Hepatol 2016;65:1217–1231 [DOI] [PubMed] [Google Scholar]
- 32. Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant 2005;5:2605–2610 [DOI] [PubMed] [Google Scholar]
- 33. Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom Ket al. . Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol 2006;30:986–993 [DOI] [PubMed] [Google Scholar]
- 34. Bogner A, Reissfelder C, Striebel F, Mehrabi A, Ghamarnejad O, Rahbari Met al. . Intraoperative increase of portal venous pressure is an immediate predictor of posthepatectomy liver failure after major hepatectomy: a prospective study. Ann Surg 2021;274:e10–e17 [DOI] [PubMed] [Google Scholar]
- 35. Reiterer C, Taschner A, Luf F, Hecking M, Tamandl D, Zotti Oet al. . Effect of liver resection-induced increases in hepatic venous pressure gradient on development of postoperative acute kidney injury. BMC Nephrol 2022;23:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology 2017;65:1384–1392 [DOI] [PubMed] [Google Scholar]
- 37. Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 2006;43:S45–S53 [DOI] [PubMed] [Google Scholar]
- 38. Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg 2009;16:145–155 [DOI] [PubMed] [Google Scholar]
- 39. Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli Vet al. . Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology 1999;29:403–411 [DOI] [PubMed] [Google Scholar]
- 40. Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 2004;5:836–847 [DOI] [PubMed] [Google Scholar]
- 41. Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol 2013;59:583–594 [DOI] [PubMed] [Google Scholar]
- 42. Schmidt SC, Hamann S, Langrehr JM, Höflich C, Mittler J, Jacob Det al. . Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepatobiliary Pancreat Surg 2007;14:484–492 [DOI] [PubMed] [Google Scholar]
- 43. Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 2003;284:G15–G26 [DOI] [PubMed] [Google Scholar]
- 44. Ortmayr G, Brunnthaler L, Pereyra D, Huber H, Santol J, Rumpf Bet al. . Immunological aspects of AXL/GAS-6 in the context of human liver regeneration. Hepatol Commun 2022;6:576–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eshkenazy R, Dreznik Y, Lahat E, Zakai BB, Zendel A, Ariche A. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg Nutr 2014;3:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martins PN, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int 2008;28:3–11 [DOI] [PubMed] [Google Scholar]
- 47. Bockamp E, Sprengel R, Eshkind L, Lehmann T, Braun JM, Emmrich Fet al. . Conditional transgenic mouse models: from the basics to genome-wide sets of knockouts and current studies of tissue regeneration. Regen Med 2008;3:217–235 [DOI] [PubMed] [Google Scholar]
- 48. Yokoyama Y, Schwacha MG, Samy TS, Bland KI, Chaudry IH. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res 2002;26:63–76 [DOI] [PubMed] [Google Scholar]
- 49. Tsugawa Y, Natori M, Handa H, Imai T. Estradiol accelerates liver regeneration through estrogen receptor α. Clin Exp Gastroenterol 2019;12:331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ku G DlC, Aizpuru M, Hackl H, Ubl DS, Habermann EB, Pery Ret al. . Hepatocellular carcinoma as predominant cancer subgroup accounting for sex differences in post-hepatectomy liver failure, morbidity and mortality. HPB (Oxford) 2022;24:1453–1463 [DOI] [PubMed] [Google Scholar]
- 51. Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab 2009;20:171–176 [DOI] [PubMed] [Google Scholar]
- 52. Furrer K, Rickenbacher A, Tian Y, Jochum W, Bittermann AG, Käch Aet al. . Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci USA 2011;108:2945–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaibori M, Inoue T, Sakakura Y, Oda M, Nagahama T, Kwon AHet al. . Impairment of activation of hepatocyte growth factor precursor into its mature form in rats with liver cirrhosis. J Surg Res 2002;106:108–114 [DOI] [PubMed] [Google Scholar]
- 54. Little SA, Jarnagin WR, DeMatteo RP, Blumgart LH, Fong Y. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg 2002;6:88–94 [DOI] [PubMed] [Google Scholar]
- 55. Bucher NL. Insulin, glucagon, and the liver. Adv Enzyme Regul 1976;15:221–230 [DOI] [PubMed] [Google Scholar]
- 56. Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med 1994;331:1547–1552 [DOI] [PubMed] [Google Scholar]
- 58. Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol 2012;19:4287–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reddy SK, Marsh JW, Varley PR, Mock BK, Chopra KB, Geller DAet al. . Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case-control study. Hepatology 2012;56:2221–2230 [DOI] [PubMed] [Google Scholar]
- 60. Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SRet al. . Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol 2008;34:782–786 [DOI] [PubMed] [Google Scholar]
- 61. Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak Pet al. . Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol 2010;28:2549–2555 [DOI] [PubMed] [Google Scholar]
- 62. Capussotti L, Muratore A, Amisano M, Polastri R, Bouzari H, Massucco P. Liver resection for hepatocellular carcinoma on cirrhosis: analysis of mortality, morbidity and survival–a European single center experience. Eur J Surg Oncol 2005;31:986–993 [DOI] [PubMed] [Google Scholar]
- 63. Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JCet al. . Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018–1022 [DOI] [PubMed] [Google Scholar]
- 64. Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg 1992;163:515–518 [DOI] [PubMed] [Google Scholar]
- 65. Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto Jet al. . Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993;18:79–85 [PubMed] [Google Scholar]
- 66. Cherqui D, Benoist S, Malassagne B, Humeres R, Rodriguez V, Fagniez PL. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg 2000;135:302–308 [DOI] [PubMed] [Google Scholar]
- 67. Makino H, Shimizu H, Ito H, Kimura F, Ambiru S, Togawa Aet al. . Changes in growth factor and cytokine expression in biliary obstructed rat liver and their relationship with delayed liver regeneration after partial hepatectomy. World J Gastroenterol 2006;12:2053–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Otao R, Beppu T, Isiko T, Mima K, Okabe H, Hayashi Het al. . External biliary drainage and liver regeneration after major hepatectomy. Br J Surg 2012;99:1569–1574 [DOI] [PubMed] [Google Scholar]
- 69. Koelfat KVK, van Mierlo KMC, Lodewick TM, Bloemen JG, van der Kroft G, Amygdalos Iet al. . Bile salt and FGF19 signaling in the early phase of human liver regeneration. Hepatol Commun 2021;5:1400–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen X, Zhai J, Cai X, Zhang Y, Wei L, Shi Let al. . Severity of portal hypertension and prediction of postoperative liver failure after liver resection in patients with Child-Pugh grade A cirrhosis. Br J Surg 2012;99:1701–1710 [DOI] [PubMed] [Google Scholar]
- 71. Golse N, Joly F, Combari P, Lewin M, Nicolas Q, Audebert Cet al. . Predicting the risk of post-hepatectomy portal hypertension using a digital twin: a clinical proof of concept. J Hepatol 2021;74:661–669 [DOI] [PubMed] [Google Scholar]
- 72. Cucchetti A, Cescon M, Golfieri R, Piscaglia F, Renzulli M, Neri Fet al. . Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J Hepatol 2016;64:79–86 [DOI] [PubMed] [Google Scholar]
- 73. Bismuth H, Majno PE. Hepatobiliary surgery. J Hepatol 2000;32:208–224 [DOI] [PubMed] [Google Scholar]
- 74. Li C, Mi K, Wen TF, Yan LN, Li B. Safety of patients with a graft to body weight ratio less than 0.8 per cent in living donor liver transplantation using right hepatic lobe without middle hepatic vein. Hepatogastroenterology 2012;59:469–472 [DOI] [PubMed] [Google Scholar]
- 75. Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano Ket al. . One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198–1206; discussion 206 [DOI] [PubMed] [Google Scholar]
- 76. Fagenson AM, Gleeson EM, Nabi F, Lau KN, Pitt HA. When does a Pringle maneuver cause harm? HPB (Oxford) 2021;23:587–594 [DOI] [PubMed] [Google Scholar]
- 77. Fujii Y, Shimada H, Endo I, Morioka D, Nagano Y, Miura Yet al. . Risk factors of posthepatectomy liver failure after portal vein embolization. J Hepatobiliary Pancreat Surg 2003;10:226–232 [DOI] [PubMed] [Google Scholar]
- 78. Savlid M, Strand AH, Jansson A, Agustsson T, Söderdahl G, Lundell Let al. . Transection of the liver parenchyma with an ultrasound dissector or a stapler device: results of a randomized clinical study. World J Surg 2013;37:799–805 [DOI] [PubMed] [Google Scholar]
- 79. Schwarz C, Klaus DA, Tudor B, Fleischmann E, Wekerle T, Roth Get al. . Transection speed and impact on perioperative inflammatory response—a randomized controlled trial comparing stapler hepatectomy and CUSA resection. PloS One 2015;10:e0140314-e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pan Y, Xia S, Cai J, Chen K, Cai X. Efficacy of laparoscopic hepatectomy versus open surgery for hepatocellular carcinoma with cirrhosis: a meta-analysis of case-matched studies. Front Oncol 2021;11:652272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu X, Huang Z, Lau WY, Li W, Lin P, Zhang Let al. . Perioperative and long-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with well-preserved liver function and cirrhotic background: a propensity score matching study. Surg Endosc 2019;33:206–215 [DOI] [PubMed] [Google Scholar]
- 82. Casellas-Robert M, Lim C, Lopez-Ben S, Llado L, Salloum C, Codina-Font Jet al. . Laparoscopic liver resection for hepatocellular carcinoma in Child-Pugh A patients with and without portal hypertension: a multicentre study. World J Surg 2020;44:3915–3922 [DOI] [PubMed] [Google Scholar]
- 83. Cipriani F, Fantini C, Ratti F, Lauro R, Tranchart H, Halls Met al. . Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc 2018;32:617–626 [DOI] [PubMed] [Google Scholar]
- 84. Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg 2012;29:6–17 [DOI] [PubMed] [Google Scholar]
- 85. Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya Tet al. . Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999;188:304–309 [DOI] [PubMed] [Google Scholar]
- 86. Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LHet al. . Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7:325–330 [DOI] [PubMed] [Google Scholar]
- 87. Van Thiel DH, Hagler NG, Schade RR, Skolnick ML, Heyl AP, Rosenblum Eet al. . In vivo hepatic volume determination using sonography and computed tomography. Validation and a comparison of the two techniques. Gastroenterology 1985;88:1812–1817 [DOI] [PubMed] [Google Scholar]
- 88. Ribero D, Amisano M, Bertuzzo F, Langella S, Lo Tesoriere R, Ferrero Aet al. . Measured versus estimated total liver volume to preoperatively assess the adequacy of the future liver remnant: which method should we use? Ann Surg 2013;258:801–806, discussion 6–7 [DOI] [PubMed] [Google Scholar]
- 89. Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol 2008;25:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Olthof PB, van Dam R, Jovine E, Campos RR, de Santibanes E, Oldhafer Ket al. . Accuracy of estimated total liver volume formulas before liver resection. Surgery 2019;166:247–253 [DOI] [PubMed] [Google Scholar]
- 91. Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst Oet al. . Remnant liver volume to body weight ratio > or = 0.5 per cent: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg 2007;204:22–33 [DOI] [PubMed] [Google Scholar]
- 92. Pulitano C, Crawford M, Joseph D, Aldrighetti L, Sandroussi C. Preoperative assessment of postoperative liver function: the importance of residual liver volume. J Surg Oncol 2014;110:445–450 [DOI] [PubMed] [Google Scholar]
- 93. Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1271–1280 [DOI] [PubMed] [Google Scholar]
- 94. Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJet al. . Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–548 [DOI] [PubMed] [Google Scholar]
- 95. Takahashi T, Togo S, Tanaka K, Endo I, Fujii Y, Shimada H. Safe and permissible limits of hepatectomy in obstructive jaundice patients. World J Surg 2004;28:475–481 [DOI] [PubMed] [Google Scholar]
- 96. Olthof PB, Coelen RJS, Bennink RJ, Heger M, Lam MF, Besselink MGet al. . (99 m)Tc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford) 2017;19:850–858 [DOI] [PubMed] [Google Scholar]
- 97. Suda K, Ohtsuka M, Ambiru S, Kimura F, Shimizu H, Yoshidome Het al. . Risk factors of liver dysfunction after extended hepatic resection in biliary tract malignancies. Am J Surg 2009;197:752–758 [DOI] [PubMed] [Google Scholar]
- 98. Tu R, Xia LP, Yu AL, Wu L. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol 2007;13:3956–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yoshino K, Yoh T, Taura K, Seo S, Ciria R, Briceno-Delgado J. A systematic review of prediction models for post-hepatectomy liver failure in patients undergoing liver surgery. HPB (Oxford) 2021;23:1311–1320 [DOI] [PubMed] [Google Scholar]
- 100. Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Digest Surg 2012;29:6–17 [DOI] [PubMed] [Google Scholar]
- 101. Rassam F, Olthof PB, van Lienden KP, Bennink RJ, Erdmann JI, Swijnenburg RJet al. . Comparison of functional and volumetric increase of the future remnant liver and postoperative outcomes after portal vein embolization and complete or partial associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Transl Med 2020;8:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fagenson AM, Gleeson EM, Pitt HA, Lau KN. Albumin-bilirubin score vs model for end-stage liver disease in predicting post-hepatectomy outcomes. J Am Coll Surg 2020;230:637–645 [DOI] [PubMed] [Google Scholar]
- 103. Wong JSW, Wong GLH, Chan AWH, Wong VWS, Cheung YS, Chong CNet al. . Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg 2013;257:922–928 [DOI] [PubMed] [Google Scholar]
- 104. Grainger SL, Keeling PWN, Brown IMH, Marigold JH, Thompson RPH. Clearance and non-invasive determination of the hepatic extraction of indocyanine green in baboons and man. Clin Sci 1983;64:207–212 [DOI] [PubMed] [Google Scholar]
- 105. Ekman M, Fjalling M, Friman S, Carlson S, Volkmann R. Liver uptake function measured by IODIDA clearance rate in liver transplant patients and healthy volunteers. Nucl Med Commun 1996;17:235–242 [DOI] [PubMed] [Google Scholar]
- 106. Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford) 2010;12:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Haegele S, Reiter S, Wanek D, Offensperger F, Pereyra D, Stremitzer Set al. . Perioperative non-invasive indocyanine green-clearance testing to predict postoperative outcome after liver resection. PLoS ONE 2016;11:e0165481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rassam F, Olthof PB, Richardson H, van Gulik TM, Bennink RJ. Practical guidelines for the use of technetium-99 m mebrofenin hepatobiliary scintigraphy in the quantitative assessment of liver function. Nucl Med Commun 2019;40:297–307 [DOI] [PubMed] [Google Scholar]
- 109. Kubota Y, Kitagawa S, Inoue K, Hakawa SK, Kojima M, Tanaka Y. Hepatic functional scintigraphic imaging with tc-99 m galactosyl serum-albumin. Hepato-Gastroenterol 1993;40:32–36 [PubMed] [Google Scholar]
- 110. Wang Q, Kesen S, Liljeroth M, Nilsson H, Zhao Y, Sparrelid Eet al. . Quantitative evaluation of liver function with gadoxetic acid enhanced MRI: comparison among signal intensity-, T1-relaxometry-, and dynamic-hepatocyte-specific-contrast-enhanced MRI- derived parameters. Scand J Gastroenterol 2022;57:705–712 [DOI] [PubMed] [Google Scholar]
- 111. Notake T, Shimizu A, Kubota K, Ikehara T, Hayashi H, Yasukawa Ket al. . Hepatocellular uptake index obtained with gadoxetate disodium-enhanced magnetic resonance imaging in the assessment future liver remnant function after major hepatectomy for biliary malignancy. BJS Open 2021;5:zraa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Evans R, Taylor S, Janes S, Halligan S, Morton A, Navani Net al. . Patient experience and perceived acceptability of whole-body magnetic resonance imaging for staging colorectal and lung cancer compared with current staging scans: a qualitative study. BMJ Open 2017;7:e016391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. de Graaf W, van Lienden KP, Dinant S, Roelofs JJTH, Busch ORC, Gouma DJet al. . Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg 2010;14:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gupta M, Choudhury PS, Singh S, Hazarika D. Liver functional volumetry by tc-99 m mebrofenin hepatobiliary scintigraphy before major liver resection: a game changer. Indian J Nucl Med 2018;33:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Serenari M, Bonatti C, Zanoni L, Peta G, Tabacchi E, Cucchetti Aet al. . The role of hepatobiliary scintigraphy combined with spect/CT in predicting severity of liver failure before major hepatectomy: a single-center pilot study. Updates Surg 2021;73:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepato-Biliary-Pancreat Surg 2007;14:159–166 [DOI] [PubMed] [Google Scholar]
- 117. Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg 2001;88:165–175 [DOI] [PubMed] [Google Scholar]
- 118. Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson Jet al. . Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49–57 [DOI] [PubMed] [Google Scholar]
- 119. van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TMet al. . Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain Vet al. . Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Olthof PB, Aldrighetti L, Alikhanov R, Cescon M, Groot Koerkamp B, Jarnagin WRet al. . Portal vein embolization is associated with reduced liver failure and mortality in high-risk resections for perihilar cholangiocarcinoma. Ann Surg Oncol 2020;27:2311–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Yamaguchi Jet al. . The predictive value of indocyanine green clearance in future liver remnant for posthepatectomy liver failure following hepatectomy with extrahepatic bile duct resection. World J Surg 2016;40:1440–1447 [DOI] [PubMed] [Google Scholar]
- 123. Franken LC, Rassam F, van Lienden KP, Bennink RJ, Besselink MG, Busch ORet al. . Effect of structured use of preoperative portal vein embolization on outcomes after liver resection of perihilar cholangiocarcinoma. BJS Open 2020;4:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Omichi K, Yamashita S, Cloyd JM, Shindoh J, Mizuno T, Chun YSet al. . Portal vein embolization reduces postoperative hepatic insufficiency associated with postchemotherapy hepatic atrophy. J Gastrointest Surg 2018;22:60–67 [DOI] [PubMed] [Google Scholar]
- 125. Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine Aet al. . Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg 2000;232:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent Aet al. . Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000;231:480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology 1996;24:1386–1391 [DOI] [PubMed] [Google Scholar]
- 128. Shindoh J, Tzeng CW, Aloia TA, Curley SA, Zimmitti G, Wei SHet al. . Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br J Surg 2013;100:1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Leung U, Simpson AL, Araujo RL, Gonen M, McAuliffe C, Miga MIet al. . Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg 2014;219:620–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf Pet al. . One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg 2003;185:221–229 [DOI] [PubMed] [Google Scholar]
- 132. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SAet al. . Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405–414 [DOI] [PubMed] [Google Scholar]
- 133. Schnitzbauer AA. A comparison of pitfalls after ALPPS stage 1 or portal vein embolization in small-for-size setting hepatectomies. Visc Med 2017;33:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sandstrom P, Rosok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell Get al. . ALPPS Improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian multicenter randomized controlled trial (LIGRO Trial). Ann Surg 2018;267:833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel Met al. . Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg 2015;262:780–785, discussion 5–6 [DOI] [PubMed] [Google Scholar]
- 136. Huiskens J, Schadde E, Lang H, Malago M, Petrowsky H, de Santibanes Eet al. . Avoiding postoperative mortality after ALPPS-development of a tumor-specific risk score for colorectal liver metastases. HPB (Oxford) 2019;21:898–905 [DOI] [PubMed] [Google Scholar]
- 137. Ulmer TF, de Jong C, Andert A, Bruners P, Heidenhain CM, Schoening Wet al. . ALPPS procedure in insufficient hypertrophy after portal vein embolization (PVE). World J Surg 2017;41:250–257 [DOI] [PubMed] [Google Scholar]
- 138. Sparrelid E, Gilg S, Brismar TB, Lundell L, Isaksson B. Rescue ALPPS is efficient and safe after failed portal vein occlusion in patients with colorectal liver metastases. Langenbecks Arch Surg 2017;402:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schadde E. Combined portal vein and hepatic vein embolization-finally the platinum procedure of regenerative liver surgery? Hepatobiliary Surg Nutr 2020;9:92–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Petrowsky H, Linecker M, Raptis DA, Kuemmerli C, Fritsch R, Kirimker OEet al. . First long-term oncologic results of the ALPPS procedure in a large cohort of patients with colorectal liver metastases. Ann Surg 2020;272:793–800 [DOI] [PubMed] [Google Scholar]
- 141. Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet Pet al. . Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol 2016;26:4259–4267 [DOI] [PubMed] [Google Scholar]
- 142. Laurent C, Fernandez B, Marichez A, Adam JP, Papadopoulos P, Lapuyade Bet al. . Radiological simultaneous portohepatic vein embolization (RASPE) before major hepatectomy: a better way to optimize liver hypertrophy compared to portal vein embolization. Ann Surg 2020;272:199–205 [DOI] [PubMed] [Google Scholar]
- 143. Kobayashi K, Yamaguchi T, Denys A, Perron L, Halkic N, Demartines Net al. . Liver venous deprivation compared to portal vein embolization to induce hypertrophy of the future liver remnant before major hepatectomy: a single center experience. Surgery 2020;167:917–923 [DOI] [PubMed] [Google Scholar]
- 144. Panaro F, Giannone F, Riviere B, Sgarbura O, Cusumano C, Deshayes Eet al. . Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center. Hepatobiliary Surg Nutr 2019;8:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Le Roy B, Gallon A, Cauchy F, Pereira B, Gagniere J, Lambert Cet al. . Combined biembolization induces higher hypertrophy than portal vein embolization before major liver resection. HPB (Oxford) 2020;22:298–305 [DOI] [PubMed] [Google Scholar]
- 146. Hocquelet A, Sotiriadis C, Duran R, Guiu B, Yamaguchi T, Halkic Net al. . Preoperative portal vein embolization alone with biliary drainage compared to a combination of simultaneous portal vein, right hepatic vein embolization and biliary drainage in Klatskin tumor. Cardiovasc Intervent Radiol 2018;41:1885–1891 [DOI] [PubMed] [Google Scholar]
- 147. Heil J, Schadde E. Simultaneous portal and hepatic vein embolization before major liver resection. Langenbecks Arch Surg 2021;406:1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pringle JHV. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908;48:541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Capussotti L, Muratore A, Ferrero A, Massucco P, Ribero D, Polastri R. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br J Surg 2006;93:685–689 [DOI] [PubMed] [Google Scholar]
- 150. Lee KF, Cheung YS, Wong J, Chong CC, Wong JS, Lai PB. Randomized clinical trial of open hepatectomy with or without intermittent Pringle manoeuvre. Br J Surg 2012;99:1203–1209 [DOI] [PubMed] [Google Scholar]
- 151. Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant 2011;11:1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol 2003;18:891–902 [DOI] [PubMed] [Google Scholar]
- 153. Badia JM, Ayton CL, Evans TJ, Carpenter AJ, Nawfal G, Kinderman Het al. . Systemic cytokine response to hepatic resections under total vascular exclusion. Eur J Surg 1998;164:185–190 [DOI] [PubMed] [Google Scholar]
- 154. Kim YI, Song KE, Ryeon HK, Hwang YJ, Yun YK, Lee JWet al. . Enhanced inflammatory cytokine production at ischemia/reperfusion in human liver resection. Hepatogastroenterology 2002;49:1077–1082 [PubMed] [Google Scholar]
- 155. Dello SAWG, Reisinger KW, van Dam RM, Bemelmans MHA, van Kuppevelt TH, van den Broek MAJet al. . Total intermittent Pringle maneuver during liver resection can induce intestinal epithelial cell damage and endotoxemia. Plos One 2012;7:e30539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kambakamba P, Linecker M, Schneider M, Kron P, Limani P, Tschuor Cet al. . Novel benefits of remote ischemic preconditioning through VEGF-dependent protection from resection-induced liver failure in the mouse. Ann Surg 2018;268:885–893 [DOI] [PubMed] [Google Scholar]
- 157. O'Neill S, Leuschner S, McNally SJ, Garden OJ, Wigmore SJ, Harrison EM. Meta-analysis of ischaemic preconditioning for liver resections. Br J Surg 2013;100:1689–1700 [DOI] [PubMed] [Google Scholar]
- 158. Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli Fet al. . Continuous versus intermittent portal triad clamping for liver resection—a controlled study. Ann Surg 1999;229:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fagenson AM, Gleeson EM, Nabi F, Lau KN, Pitt HA. When does a Pringle maneuver cause harm? HPB (Oxford) 2021;23:587–594 [DOI] [PubMed] [Google Scholar]
- 160. Sanjay P, Ong I, Bartlett A, Powell JJ, Wigmore SJ. Meta-analysis of intermittent Pringle manoeuvre versus no Pringle manoeuvre in elective liver surgery. Anz J Surg 2013;83:719–723 [DOI] [PubMed] [Google Scholar]
- 161. Ni JS, Lau WY, Yang Y, Pan ZY, Wang ZG, Liu Het al. . A prospective randomized controlled trial to compare Pringle manoeuvre with hemi-hepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis. J Gastrointest Surg 2013;17:1414–1421 [DOI] [PubMed] [Google Scholar]
- 162. Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Duration of hepatic vascular inflow clamping and survival after liver resection for hepatocellular carcinoma. Br J Surg 2011;98:1284–1290 [DOI] [PubMed] [Google Scholar]
- 163. Huang Y, Liao A, Pu X, Yang J, Lv T, Yan Let al. . A randomized controlled trial of effect of 15- or 25-min intermittent Pringle maneuver on hepatectomy for hepatocellular carcinoma. Surgery 2022;171:1596–1604 [DOI] [PubMed] [Google Scholar]
- 164. Melendez J, Ferri E, Zwillman M, Fischer M, DeMatteo R, Leung Det al. . Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg 2001;192:47–53 [DOI] [PubMed] [Google Scholar]
- 165. Zimmitti G, Vauthey JN, Shindoh J, Tzeng CW, Roses RE, Ribero Det al. . Systematic use of an intraoperative air leak test at the time of major liver resection reduces the rate of postoperative biliary complications. J Am Coll Surg 2013;217:1028–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ma JY, Li XF, Yan ZL, Hasan MM, Wang K, Wang Yet al. . Randomized controlled trial of hepatic portal reocclusion as a new option for detecting bile leakage during hepatic resection. Dig Surg 2017;34:328–334 [DOI] [PubMed] [Google Scholar]
- 167. Li J, Malago M, Sotiropoulos GC, Lang H, Schaffer R, Paul Aet al. . Intraoperative application of “white test” to reduce postoperative bile leak after major liver resection: results of a prospective cohort study in 137 patients. Langenbecks Arch Surg 2009;394:1019–1024 [DOI] [PubMed] [Google Scholar]
- 168. Christ B, Collatz M, Dahmen U, Herrmann KH, Hopfl S, Konig Met al. . Hepatectomy-induced alterations in hepatic perfusion and function—toward multi-scale computational modeling for a better prediction of post-hepatectomy liver function. Front Physiol 2021;12:733868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Eipel C, Abshagen K, Ritter J, Cantre D, Menger MD, Vollmar B. Splenectomy improves survival by increasing arterial blood supply in a rat model of reduced-size liver. Transpl Int 2010;23:998–1007 [DOI] [PubMed] [Google Scholar]
- 170. Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transpl 2003;9:S36–S41 [DOI] [PubMed] [Google Scholar]
- 171. Wang H, Ikegami T, Harada N, Yoshizumi T, Soejima Y, Uchiyama Het al. . Optimal changes in portal hemodynamics induced by splenectomy during living donor liver transplantation. Surg Today 2015;45:979–985 [DOI] [PubMed] [Google Scholar]
- 172. Nagao Y, Akahoshi T, Kamori M, Uehara H, Hashimoto N, Kinjo Net al. . Liver regeneration is promoted by increasing serotonin content in rat liver with secondary biliary cirrhosis. Hepatol Res 2011;41:784–794 [DOI] [PubMed] [Google Scholar]
- 173. Troisi R, Hoste E, Van Langenhove P, Decruyenaere J, Voet D, Hesse UJet al. . Modulation of liver graft hemodynamics by partial ablation of the splenic circuit: a way to increase hepatic artery flow? Transplant Proc 2001;33:1445–1446 [DOI] [PubMed] [Google Scholar]
- 174. Rhaiem R, Piardi T, Chetboun M, Pessaux P, Lestra T, Memeo Ret al. . Portal inflow modulation by somatostatin after Major liver resection: a pilot study. Ann Surg. 2018;267:e101–e103 [DOI] [PubMed] [Google Scholar]
- 175. Hammond JS, Godtliebsen F, Steigen S, Guha IN, Wyatt J, Revhaug Aet al. . The effects of terlipressin and direct portacaval shunting on liver hemodynamics following 80 per cent hepatectomy in the pig. Clin Sci (Lond) 2019;133:153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jo HS, Park HJ, Choi YY, Seok JI, Han JH, Yoon YIet al. . Portal modulation effects of terlipressin on liver regeneration and survival in a porcine model subjected to 90 per cent hepatectomy. Am J Transl Res 2021;13:5880–5891 [PMC free article] [PubMed] [Google Scholar]
- 177. Hessheimer AJ, Escobar B, Munoz J, Flores E, Gracia-Sancho J, Taura Pet al. . Somatostatin therapy protects porcine livers in small-for-size liver transplantation. Am J Transplant 2014;14:1806–1816 [DOI] [PubMed] [Google Scholar]
- 178. Kohler A, Perrodin S, De Gottardi A, Candinas D, Beldi G. Effectiveness of terlipressin for prevention of complications after major liver resection—a randomized placebo-controlled trial. HPB (Oxford) 2020;22:884–891 [DOI] [PubMed] [Google Scholar]
- 179. Jegatheeswaran S, Siriwardena AK. Experimental and clinical evidence for modification of hepatic ischaemia-reperfusion injury by N-acetylcysteine during major liver surgery. HPB (Oxford) 2011;13:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Grendar J, Ouellet JF, McKay A, Sutherland FR, Bathe OF, Ball CGet al. . Effect of N-acetylcysteine on liver recovery after resection: a randomized clinical trial. J Surg Oncol 2016;114:446–450 [DOI] [PubMed] [Google Scholar]
- 181. Robinson SM, Saif R, Sen G, French JJ, Jaques BC, Charnley RMet al. . N-acetylcysteine administration does not improve patient outcome after liver resection. HPB (Oxford) 2013;15:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Schmidt SC, Hamann S, Langrehr JM, Hoflich C, Mittler J, Jacob Det al. . Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepatobiliary Pancreat Surg 2007;14:484–492 [DOI] [PubMed] [Google Scholar]
- 183. Hayashi Y, Takayama T, Yamazaki S, Moriguchi M, Ohkubo T, Nakayama Het al. . Validation of perioperative steroids administration in liver resection: a randomized controlled trial. Ann Surg 2011;253:50–55 [DOI] [PubMed] [Google Scholar]
- 184. Hasegawa Y, Nitta H, Takahara T, Katagiri H, Kanno S, Umemura Aet al. . Glucocorticoid use and ischemia-reperfusion injury in laparoscopic liver resection: randomized controlled trial. Ann Gastroenterol Surg 2020;4:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Steinthorsdottir KJ, Awada HN, Schultz NA, Larsen PN, Hillingso JG, Jans Oet al. . Preoperative high-dose glucocorticoids for early recovery after liver resection: randomized double-blinded trial. BJS Open 2021;5:zrab063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Richardson AJ, Laurence JM, Lam VW. Use of pre-operative steroids in liver resection: a systematic review and meta-analysis. HPB (Oxford) 2014;16:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. European Association for the Study of the Liver. et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047–1081 [DOI] [PubMed] [Google Scholar]
- 188. Lafaro K, Buettner S, Maqsood H, Wagner D, Bagante F, Spolverato Get al. . Defining post hepatectomy liver insufficiency: where do we stand? J Gastrointest Surg 2015;19:2079–2092 [DOI] [PubMed] [Google Scholar]
- 189. Soreide JA, Deshpande R. Post hepatectomy liver failure (PHLF)—recent advances in prevention and clinical management. Eur J Surg Oncol 2021;47:216–224 [DOI] [PubMed] [Google Scholar]
- 190. Dasari BV, Rahman R, Khan S, Bennett D, Hodson J, Isaac Jet al. . Safety and feasibility of an enhanced recovery pathway after a liver resection: prospective cohort study. HPB (Oxford) 2015;17:700–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Garwood RA, Sawyer RG, Thompson L, Adams RB. Infectious complications after hepatic resection. Am Surg 2004;70:787–792 [PubMed] [Google Scholar]
- 192. Murtha-Lemekhova A, Fuchs J, Teroerde M, Chiriac U, Klotz R, Hornuss Det al. . Routine postoperative antibiotic prophylaxis offers no benefit after hepatectomy-a systematic review and meta-analysis. Antibiotics (Basel) 2022;11:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Saadat LV, Brajcich BC, Liu Y, Ko C, D'Angelica MI. Defining the risk of liver failure after minor hepatectomy: a NSQIP analysis of 7029 patients. HPB (Oxford) 2021;23:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Haussinger D, Dhiman RK, Felipo V, Gorg B, Jalan R, Kircheis Get al. . Hepatic encephalopathy. Nat Rev Dis Primers 2022;8:43. [DOI] [PubMed] [Google Scholar]
- 195. Watanabe Y, Tsuchiya A, Seino S, Kawata Y, Kojima Y, Ikarashi Set al. . Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med 2019;8:271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lei Z, Cheng N, Si A, Yang P, Guo G, Ma Wet al. . A novel nomogram for predicting postoperative liver failure after Major hepatectomy for hepatocellular carcinoma. Front Oncol 2022;12:817895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Iseki M, Mizuma M, Wakao S, Kushida Y, Kudo K, Fukase Met al. . The evaluation of the safety and efficacy of intravenously administered allogeneic multilineage-differentiating stress-enduring cells in a swine hepatectomy model. Surg Today 2021;51:634–650 [DOI] [PubMed] [Google Scholar]
- 198. Wang JL, Ding HR, Pan CY, Shi XL, Ren HZ. Mesenchymal stem cells ameliorate lipid metabolism through reducing mitochondrial damage of hepatocytes in the treatment of post-hepatectomy liver failure. Cell Death Dis 2021;12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Watanabe Y, Tsuchiya A, Terai S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin Mol Hepatol 2021;27:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Nevens F, Laleman W. Artificial liver support devices as treatment option for liver failure. Best Pract Res Clin Gastroenterol 2012;26:17–26 [DOI] [PubMed] [Google Scholar]
- 201. Stange J, Ramlow W, Mitzner S, Schmidt R, Klinkmann H. Dialysis against a recycled albumin solution enables the removal of albumin-bound toxins. Artif Organs 1993;17:809–813 [DOI] [PubMed] [Google Scholar]
- 202. Stange J, Mitzner SR, Risler T, Erley CM, Lauchart W, Goehl Het al. . Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif Organs 1999;23:319–330 [DOI] [PubMed] [Google Scholar]
- 203. Catalina MV, Barrio J, Anaya F, Salcedo M, Rincon D, Clemente Get al. . Hepatic and systemic haemodynamic changes after MARS in patients with acute on chronic liver failure. Liver Int 2003;23:39–43 [DOI] [PubMed] [Google Scholar]
- 204. Sen S, Mookerjee RP, Cheshire LM, Davies NA, Williams R, Jalan R. Albumin dialysis reduces portal pressure acutely in patients with severe alcoholic hepatitis. J Hepatol 2005;43:142–148 [DOI] [PubMed] [Google Scholar]
- 205. Banares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger Met al. . Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology 2013;57:1153–1162 [DOI] [PubMed] [Google Scholar]
- 206. Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse Bet al. . Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med 2013;159:522–531 [DOI] [PubMed] [Google Scholar]
- 207. Tandon R, Froghi S. Artificial liver support systems. J Gastroenterol Hepatol 2021;36:1164–1179 [DOI] [PubMed] [Google Scholar]
- 208. Wallon G, Guth C, Guichon C, Thevenon S, Gazon M, Viale JPet al. . Extracorporeal albumin dialysis in liver failure with MARS and SPAD: a randomized crossover trial. Blood Purif 2022;51:243–250 [DOI] [PubMed] [Google Scholar]
- 209. Peszynski P, Klammt S, Peters E, Mitzner S, Stange J, Schmidt R. Albumin dialysis: single pass vs. recirculation (MARS). Liver 2002;22:40–42 [DOI] [PubMed] [Google Scholar]
- 210. Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VCet al. . High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol 2016;64:69–78 [DOI] [PubMed] [Google Scholar]
- 211. Gilg S, Sparrelid E, Saraste L, Nowak G, Wahlin S, Stromberg Cet al. . The molecular adsorbent recirculating system in posthepatectomy liver failure: results from a prospective phase I study. Hepatol Commun 2018;2:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Sparrelid E, Gilg S, van Gulik TM. Systematic review of MARS treatment in post-hepatectomy liver failure. HPB (Oxford) 2020;22:950–960 [DOI] [PubMed] [Google Scholar]
- 213. Demetriou AA, BrownRS, Jr., Busuttil RW, Fair J, McGuire BM, Rosenthal Pet al. . Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–667; discussion 7–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. van de Kerkhove MP, Poyck PP, Deurholt T, Hoekstra R, Chamuleau RA, van Gulik TM. Liver support therapy: an overview of the AMC-bioartificial liver research. Dig Surg 2005;22:254–264 [DOI] [PubMed] [Google Scholar]
- 215. He YT, Qi YN, Zhang BQ, Li JB, Bao J. Bioartificial liver support systems for acute liver failure: a systematic review and meta-analysis of the clinical and preclinical literature. World J Gastroenterol 2019;25:3634–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Otsuka Y, Duffy JP, Saab S, Farmer DG, Ghobrial RM, Hiatt JRet al. . Postresection hepatic failure: successful treatment with liver transplantation. Liver Transpl 2007;13:672–679 [DOI] [PubMed] [Google Scholar]
- 217. Thorsen T, Solheim JM, Labori KJ, Line PD, Aandahl EM. Liver transplantation as a lifesaving procedure for posthepatectomy liver failure and iatrogenic liver injuries. Langenbecks Arch Surg 2019;404:301–308 [DOI] [PubMed] [Google Scholar]
- 218. Sparrelid E, Thorsen T, Sauter C, Jorns C, Stal P, Nordin Aet al. . Liver transplantation in patients with post-hepatectomy liver failure—a northern European multicenter cohort study. HPB (Oxford) 2021;24:1138–1144 [DOI] [PubMed] [Google Scholar]
- 219. Skrzypczyk C, Truant S, Duhamel A, Langlois C, Boleslawski E, Koriche Det al. . Relevance of the ISGLS definition of posthepatectomy liver failure in early prediction of poor outcome after liver resection: study on 680 hepatectomies. Ann Surg 2014;260:865–870; discussion 70 [DOI] [PubMed] [Google Scholar]
- 220. Harrison EM, O'Neill S, Wigmore SJ, James Garden O. Comparison of binary predictive scoring systems of posthepatectomy liver failure. Ann Surg 2017;265:e56–e57 [DOI] [PubMed] [Google Scholar]
- 221. Skrzypczyk C, Truant S, Duhamel A, Langlois C, Boleslawski E, Hebbar Met al. . Comparison of binary predictive scoring systems of posthepatectomy liver failure: a response. Ann Surg 2017;265:e57–e58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is narrative review and no data have been generated by the authors.