Figure 5.
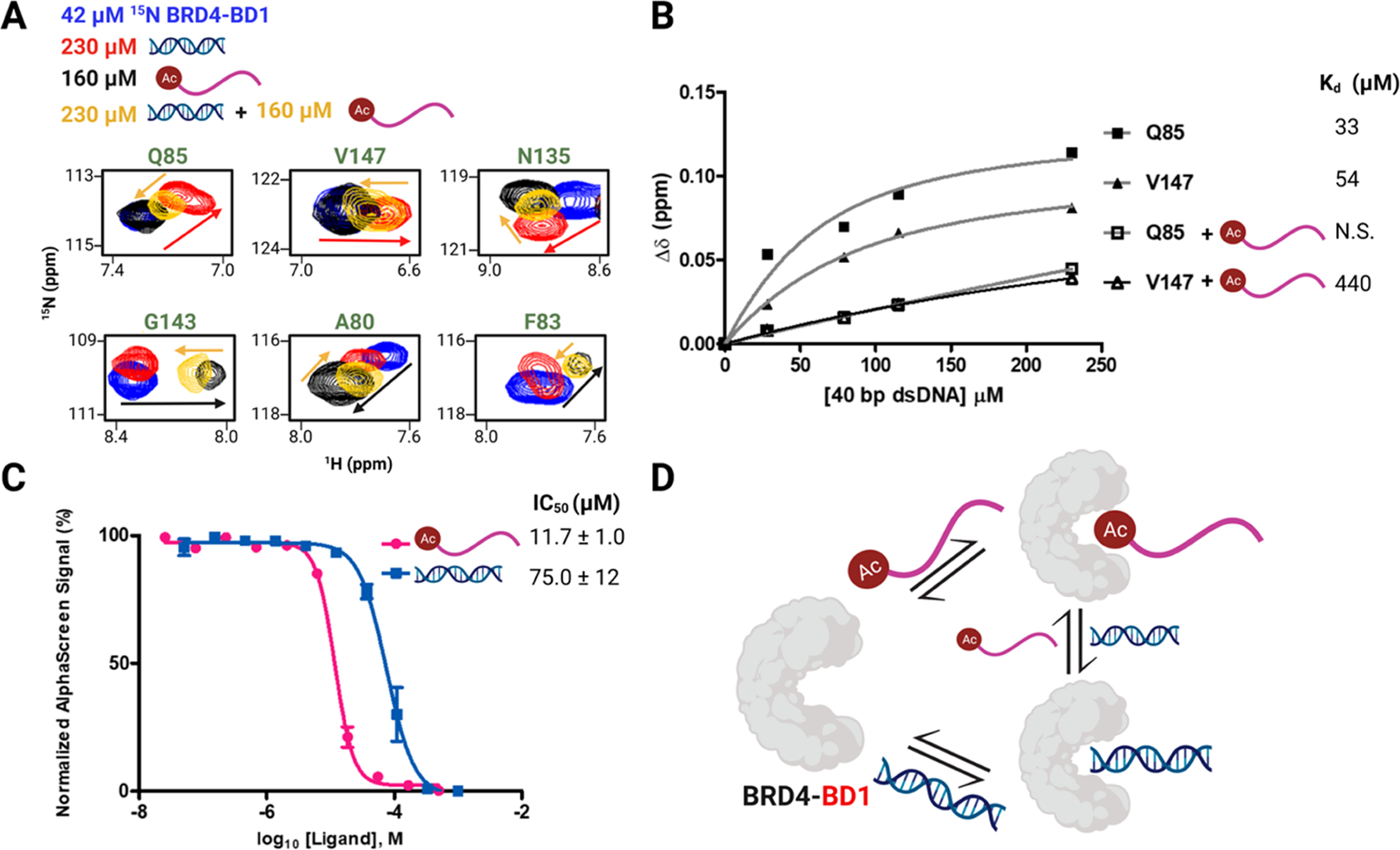
Acetylated histone and dsDNA exhibit competitive binding to BRD4–BD1. (A) Subset of residues showing the overlay of 1H–15N HSQC NMR spectra of 42 μM 15N BRD4–BD1 (blue) and in the presence of 230 μM 40 bp dsDNA (red), 160 μM H4 K5Ac,K8Ac,K12Ac,K16Ac (black), or both 230 μM 40 bp dsDNA and 160 μM H4 K5Ac,K8Ac,K12Ac,K16Ac (yellow). Arrows trace the linear trajectory between apo and histone-bound BRD4–BD1 (black) or apo and DNA-bound BRD4–BD1 (red) and upon titration of dsDNA into histone-bound BRD4–BD1 (yellow) and vice-versa. (B) Binding isotherms of residues Q85 and V147 in the absence and presence of 160 μM H4 K5Ac,K8Ac,K12Ac,K16Ac and corresponding Kd values (N.S. stands for nonsaturating). (C) AlphaScreen competition experiments with 9xHis–BRD4–BD1 using a biotinylated histone peptide reported as mean ± SD of three experimental replicates performed in duplicate. (D) Cartoon depicting the competitive binding between the acetylated histone and dsDNA to interact with BRD4–BD1.
