Abstract
Background:
In previous studies, a new Trichinella spiralis serine protease 1.1 (TsSP1.1) was identified in surface proteins of T. spiralis muscle larvae (ML) by proteomics analysis, but its functions in T. spiralis infection are unknown. The aim of this study was to investigate the roles of TsSP1.1 during larval intrusion of gut epithelium.
Methods:
From January 2019 to March 2021, complete TsSP1.1 cDNA sequence was cloned and expressed in Escherichia coli BL21 at the Department of Parasitology, Medical College of Zhengzhou University, Zhengzhou, China. Expression and location of TsSP1.1 in the parasite were investigated using indirect immunofluorescence assay (IIFA) and Western blotting. The in vitro intestinal epithelium cells (IECs) intrusion assay was used to ascertain the roles of TsSP1.1 during larval intrusion of IECs and gut epithelium.
Results:
TsSP1.1 was a surface and secretory protein, which was expressed at various T. spiralis stages, and principally localized at cuticle, stichosome and embryos of the nematode. rTsSP1.1 accelerated larval intrusion of IECs, whereas anti-rTsSP1.1 antibodies impeded larval intrusion. The acceleration and inhibtion was dose-dependently related to rTsSP1.1 and anti-TsSP1.1 antibodies. Block of the IIL with anti-rTsSP1.1 serum also impeded larval intrusion of gut mucosa.
Conclusion:
TsSP1.1 participates in T. spiralis intrusion of gut epithelium.
Keywords: Trichinella spiralis, Serine protease 1.1, Intrusion, Intestinal epithelium
Introduction
Trichinella spiralis is an important foodborne nematode worldwide (1). T. spiralis infection in humans is principally resulted from eating raw or under-cooked meat infected with infectious encapsulated muscle larvae (ML) (2). Following ingestion, T. spiralis ML in infected meat are liberated from their capsules under digestion of host’ gastric pepsin and activated to intestinal infectious larvae (IIL), the IIL penetrate into gut mucosal epithelia where they develop into adult worms (AW). Gut epithelium is the prior natural defense against IIL intrusion and prime interaction position between IIL and host. However, the mechanism of gut epithelium intrusion by the IIL is unclear. Characterization of Trichinella invasive proteins will be valuable to understand the invasive mechanism and to study vaccines for blocking Trichinella invasion.
In the lifecycle of T. spiralis, excretion/secretion (ES) protein of ML and IIL are first exposed to host intestinal epithelium cells (IECs), and they might play a major act for IECs intrusion and elicit early immune response. When the IIL were co-cultivated with IEC monolayer, the IIL intruded into the monolayer and produced various serine proteases, which entered into the IECs. By using proteomics/immunoproteomics, various serine proteases have been identified in ES or surface proteins of T. spiralis ML and IIL. Serine protease is a superfamily of proteolytic enzymes, and may play the important role in the process of Trichinella infection. Serine proteases might be the promising vaccine target molecules against intestinal Trichinella infection.
Some serine proteases participated in the IEC intrusion. However, immunization of mice with recombinant single serine protease only elicited a partial protective immunity against T. spiralis challenge. Therefore, it is needful to characterize other new T. spiralis serine proteases and ascertain their role in intruding IECs. In our previous studies, a new T. spiralis serine protease 1.1 (TsSP1.1; GenBank: ACA28930.1) was identified by proteomics analysis of T. spiralis ML surface proteins (3), but its biological properties and functions in T. spiralis infection are unknown.
The aim of this study was to clone and express recombinant TsSP1.1 (rTsSP1.1) and evaluate its roles in T. spiralis intrusion of gut epithelium.
Materials and Methods
Ethics statement
All animal experimental procedures were approved by the Life Science Ethics Committee of Zhengzhou University, China (No. SCXK 2017-0001).
Parasites, experimental animals
T. spiralis isolate (ISS534) used in this study was collected from an infected domestic pig in central China (4). Female BALB/c mice with 5 weeks old were bought from Henan Provincial Experimental Animal Center.
Worm collection and antigen preparation
Five T. spiralis-infected mouse carcasses at 42 days post infection (dpi) were artificially digested to collect the ML (5). Ten mice were infected orally with 6000 ML, euthanized at 6 hpi, and the IIL were collected from small intestine. Twenty mice were inoculated orally with 4000 ML, euthanized at 3 and 6 dpi respectively, the AW were collected from intestine. Female adults at 6 dpi were cultivated in RPMI-1640 with 10% fetal bovine serum (FBS; Gibco) at 37 °C in 5% CO2 for 24 h, the newborn larvae (NBL) were recovered. T. spiralis ML somatic crude antigens, ML ES antigens were prepared as described before. Briefly, the ML were submitted to 5 cycles of freezing-thawing, homogenized in a glass tissue grinder, and the larval fragments were further homogenized by ultrasonication (99 times 3-s cycle, 100 W, 0 °C). The supernatant containing crude proteins was collected after centrifugation at 15,000 g for 1 h at 4 °C. The ML were washed using sterile saline, cultured in RPMI-1640 medium (5000 worms/ml) at 37°C, 5% CO2 for 18 h. The culture media containing ML ES proteins were filtered with a 0.22 μm membrane, and concentrated with an ultrafiltration tube. The concentration of the ML crude and ES proteins were assessed by Bradford method.
Bioinformatics analysis of TsSP1.1
Complete TsSP1.1 cDNA sequence was retrieved from GenBank (GenBank: ACA28930.1). Physicochemical characteristics of TsSP1.1 were analyzed by bioanalysis software and websites. Tertiary structure of TsSP1.1 was predicted by PyMOL software, and its functional sites were analyzed using CN3D software (6). Amino acid sequence of TsSP1.1 was compared with serine proteases from other organisms with BioEdit (7). The GenBank accession numbers of serine proteases from Trichinella species/genotypes were as follows: T. murrelli (KRX47708.1), T. britovi (KRY58837.1), Trichinella T6 (KRX77113.1), T. patagoniensis (KRY23879.1), T. nativa (KRZ59614.1), Trichinella T8 (KRZ96357.1), T. nelsoni (KRX25899.1), Trichinella T9 (KRX62546.1), T. pseudospiralis (KRZ43046.1), T. zimbabwensis (KRZ09984.1) and T. papuae (KRZ79038.1). Phylogenetic analysis was performed with MEGA 7.0 based on the Neighbor-joining (NJ) method.
Cloning, expression and identification of rTsSP1.1
Trizol (Invitrogen, USA) was used to isolate larval cDNA of the ML, and BamH I and Pst I (shadowed) were used as restriction sites to design TsSP1.1-specific primers (5′-ATGGATCCGAAGAATGTG-GAAAACCCTAT-′; 5′-GCGCGGCTGCAGTTATAAAG TAATCTTTAAAATTTC-′). Complete TsSP1.1 cDNA sequence was amplified by PCR. The PCR products were cloned into pQE-80L (Novagen, USA), and recombinant pQE-80L/TsSP1.1 was transformed into E. coli BL21 (Novagen). Expression of rTsSP1.1 was induced with 1 mM IPTG at 30 °C for 5 h, A Ni-NTA-Sefinose resin containing His tag (Sangon Biotech, Shanghai, China) was used to purify rTsSP1.1 (8). Expression of rTsSP1.1 protein was analyzed by SDS-PAGE.
Preparation of anti-rTsSP1.1 antibodies and Western blotting analysis
Twenty mice were immunized subcutaneously with 20-μg rTsSP1.1 mixed with complete Freund’s adjuvant. Boost immunization was performed two times with 20 μg rTsSP1.1 mixed with incomplete Freund’s adjuvant at a 2-week interval (9). Two weeks after last immunization, tail blood of immunized mice was collected to isolate anti-rTsSP1.1 immune sera (10).
T. spiralis ML somatic crude antigens, ML ES antigens and rTsSP1.1 were separated by 12% SDS-PAGE. The proteins were transferred onto nitrocellulose membrane (Milli-pore, USA). The membrane was blocked with 5% nonfat milk diluted in TBST at 37 °C for 2 h and cut into strips. The strips were probed with 1:100 dilutions of various sera at 37 °C for 2 h. After washes with TBST, the strips were incubated with HRP-conjugated anti-mouse IgG (1:10000; Southern Biotech, USA) at 37 °C for 1 h. After being washed again, the strips were developed with an AEC peroxidase substrate (Solarbio, Beijing, China)
Indirect immunofluorescence assay (IIFA)
Complete worms of diverse T. spiralis developmental stages (ML, 6h and 12h IIL, 3d and 6d AW, and NBL) were fixed with 4% formaldehyde for 30 min. The fixed worms were embedded in paraffin and cut into a 2-μm thick cross-section with a microtome. Expression and tissue location of native TsSP1.1 in diverse stage worms was observed using IIFA. Complete worms and their cross-sections were blocked with 5% normal goat serum for 2 h, and then incubated at 37 °C for 2 h with 1:10 diluted various sera. Following washes with PBS, the worms were stained by goat anti-mouse IgG-FITC conjugate (1:100; Santa Cruz, USA). After washes again, complete worms and cross-sections were observed under fluorescence microscopy (11).
The in vitro IECs intrusion assay
To assess the TsSP1.1 role in the IIL intrusion of gut epithelium, the in vitro IECs intrusion test was conducted as reported before (12). In brief, 100 IIL suspended in semisolid medium were added to the IECs monolayer. The medium was pre-mixed with various doses of rTsSP1.1 or dilutions (1:100-1:1000) of various sera. After being cultured at 37 °C for 2 h, larva intrusion of IECs was examined on microscopy. The IIL invaded into the IECs and migrated was assessed as intruded larvae, whereas the worms that still suspended on IECs surface and remained in coil or spiral form were counted as non-intruded ones.
Challenge of mice with the ML incubated with anti-rTsSP1.1 serum
To evaluate the role of anti-rTsSP1.1 antibodies to block larval intrusion of intestine mucosa, 30 mice were divided into 3 groups (10 mice per group). Each mouse was infected orally with 500 ML, which were pre-incubated, with 1:100 dilutions of anti-rTsSP1.1 serum at 37 °C for 2 h. The infected mice were euthanized at 5 dpi, intestinal AWs were recovered and their sizes were examined under microscopy.
Statistical analysis
The data were analyzed by SPSS 21.0 software (IBM Corp., Armonk, NY, USA) and showed as mean ± standard deviation (SD). One-way ANOVA was used to analyze larval intrusion, intestinal adult burdens and lengths among various groups. P <0.05 was assessed as statistical significance.
Results
Bioinformatics analysis of TsSP1.1
Complete TsSP1.1 cDNA sequence is 1424 bp encoding 453 aa, with a molecular weight (MW) of 51 kDa and isoelectric point (pI) of 6.25. TsSP1.1 has a signal peptide at 1–27 aa. Subcellular location suggested that TsSP1.1 is a secretory protein. TsSP1.1 has an obvious hydrophobic region at the N-terminus, which belongs to serine protease. The homology comparison of TsSP1.1 amino acid sequences with serine protease of other species/genotypes of the genus Trichinella was shown in Fig. 1. The amino acid sequences of the TsSP1.1 had an identity of 94–95% with serine protease of eight encapsulated Trichinella species, and it had an identity of 79–81% of three non-encapsulated species.
Fig. 1:
Sequence alignment of T. spiralis serine protease 1.1 (TsSP1.1; ACA28930.1) with other Trichinella species/genotypes. BioEdit was used to analyze the sequences, distinct differences were observed in various Trichinella species/genotypes. Black shade indicated that residues identical to TsSP1.1, and grey shade showed the conservative substitutions
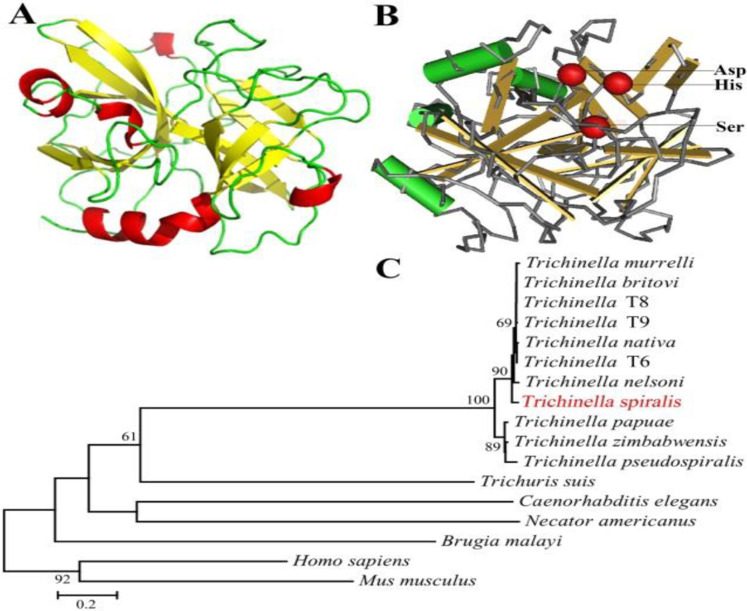
The structure prediction showed that TsSP1.1 has 5 α-helices and 11 β-strands, and a function domain of serine protease family. In three-dimensional model, the catalytic triad Aspartate-Serine–Histidine motif formed a functional domain with substrate binding sites (Fig. 2A, B). Phylogenetic tree showed that a monophyletic group of the genus Trichinella was well supported. Within the genus Trichinella, two clear clades were present: one was the clade of 8 encapsulated species/gene types, and the other was the clade of three non-encapsulated species (Fig. 2C).
Fig. 2:
Predicted three-dimensional model of TsSP1.1 and phylogenetic trees of serine proteases of 17 organisms. A: Predicted three-dimensional structure of TsSP1.1, there are 5 α-helixes (in red), 11 β-strand (in yellow), and 15 irregular coils (in green). B: Three serine protease-specific enzyme active sites (Asp, Ser and His) of TsSP1.1 are marked red. C: Phylogenetic tree of serine proteases from 17 organisms calculated using MEGA with NJ method
Expression and identification of rTsSP1.1 protein
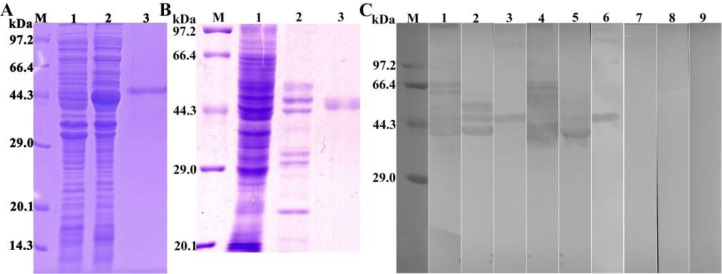
The SDS-PAGE results showed that the BL21 bacteria carrying pQE-80L/TsSP1.1 expressed a fusion protein with of 51 kDa. The MW of the rTsSP1.1 protein was consistent with the predicted combined size of TsSP1.1 (Fig. 3A). Western blotting analysis showed that rTsSP1.1 was recognized by infection serum and anti-rTsSP1.1 serum (Fig. 3C). Natural TsSP1.1 with 38.9–76.6 kDa in ML crude antigens and ML ES antigens were also identified by anti-rTsSP1.1 serum and infection serum, but not by normal serum, suggesting that natural TsSP1.1 is a secretory protein.
Fig. 3:
Identification of rTsSP1.1. A: SDS-PAGE analysis of rTsSP1.1. Lane M: Protein marker; Lane 1: ly-sate of recombinant pQE-80L/TsSP1.1 prior to induction; Lane 2: lysate of recombinant pQE-80L/TsSP1.1 following induction; Lane 3: purified rTsSP1.1. B: SDS-PAGE analysis of muscle larva (ML) somatic crude antigens, ML excretion/secretion (ES) antigens and rTsSP1.1. Lane M: protein marker; Lane 1: ML crude antigens; Lane 2: ML ES antigens; Lane 3: rTsSP1.1. C: Western blotting of rTsSP1.1. ML crude antigens (lane 1), ML ES antigens (lane 2), and rTsSP1.1 (lane 3) were recognized by infected sera. Native TsSP1.1 in ML crude antigens (lane 4), ML ES antigens (lane 5) and rTsSP1.1 (lane 6) were identified by anti-rTsSP1.1 serum; but native TsSP1.1 in ML crude antigens (lane 7), ML ES antigens (lane 8) and rTsSP1.1 (lane 9) were not recognized by normal serum
Expression and worm localization of native TsSP1.1 in various T. spiralis stages
The results of IIFA with complete worms showed that green fluorescence on surface of various T. spiralis stages (ML, IIL, AW and NBL) was seen by anti-rTsSP1.1 serum (Fig. 4). When worm cross-sections were incubated with anti-rTsSP1.1 serum, immunostaining was localized at cuticle of ML and IIL, and around embryo of female adults of the parasite (Fig. 5).
Fig. 4:
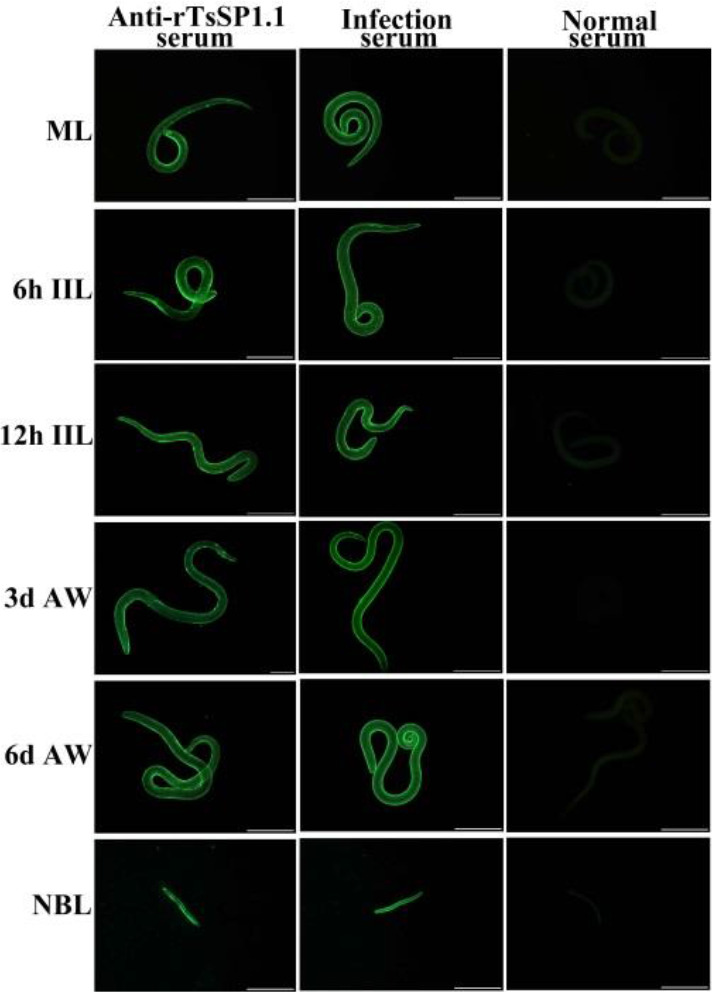
Expression of TsSP1.1 on surface of various T. spiralis phases by indirect immunofluorescence assay (IIFA). Whole worms were incubated with anti-rTsSP1.1 serum, and green fluorescence staining was observed at surface of muscle larvae (ML), intestinal infectious larvae (IIL), adult worms (AW) and newborn larvae (NBL). But normal murine serum did not recognize any worm components of the nematode. Scale bars: 100 μm
Fig. 5:
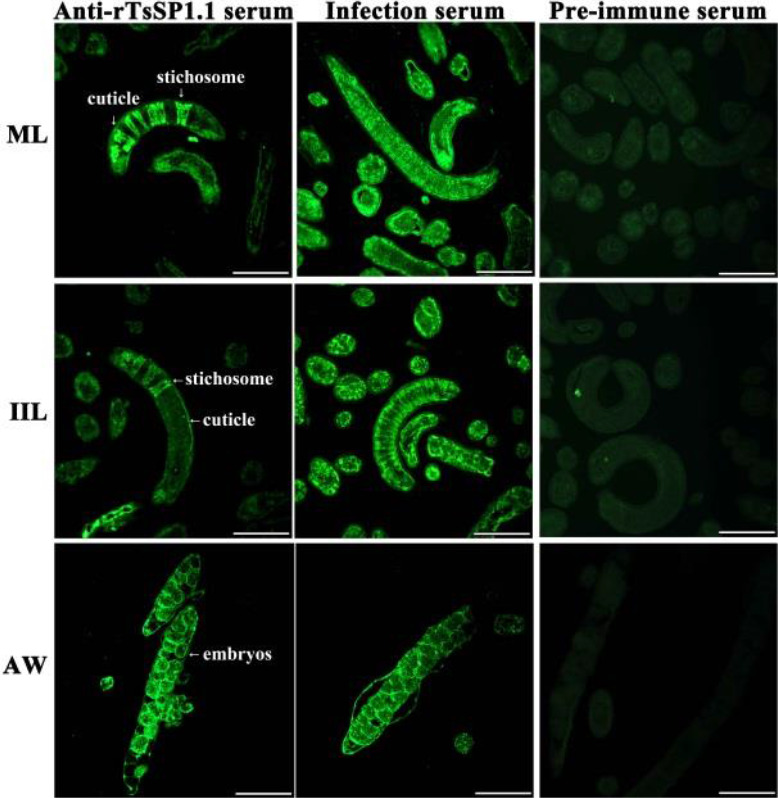
Immunolocalization of TsSP1.1 in cross-sections of various T. spiralis phases using IIFA. Green fluorescence was observed at cuticle, stichosome and embryos of the nematode. No immunostaining in worm cross-sections was seen with normal serum. Scale-bars: 100 μm
rTsSP1.1 facilitation or anti-rTsSP1.1 serum suppression on larval invasion of IECs
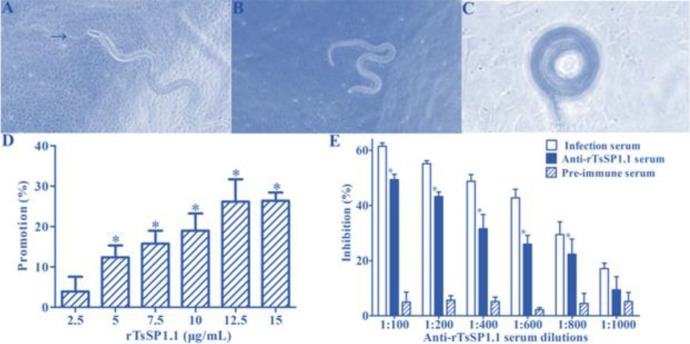
After cultivation with IECs monolayer for 2 h, the IIL that intruded into the monolayer were seen (Fig. 6A-C). When the medium was supplemented by rTsSP1.1 and cultivated with the IIL for 2 h, an evidently promotion of rTsSP1.1 on larval intrusion of IECs was observed, this promotion was rTsSP1.1 dose-dependent (r = 0.9389), and showed an elevating trend with increase of TsSP1.1 dose (F = 23.4647, P < 0.0001) (Fig. 6D). When diluted anti-rTsSP1.1 serum was added into culture medium, anti-rTsSP1.1 serum (1:100–1:800) significantly inhibited on larval intrusion compared to the PBS group (P < 0.01). The inhibition was dose-dependent of anti-rTsSP1.1 antibodies (r = 0.9598) and showed a declining trend with enlargement of serum dilutions (F = 39.5876, P < 0.0001) (Fig. 6E). Furthermore, pre-immune serum did not exhibit any inhibitory roles on larval intrusion of IECs.
Fig. 6:
rTsSP1.1 promotion or anti-rTsSP1.1 serum inhibition on intestinal epithelium cells (IECs) intrusion. A: The IIL intruded IEC monolayer were active and migratory. B: Non-intruded IIL spiraled on IEC mono-layer surface. C: Non-intruded IIL coiled on C2C12 surface. D: rTsSP1.1 accelerated IIL intrusion of IECs. E: anti-rTsSP1.1 antibodies inhibited IIL invasion of IECs. * P < 0.0001 compared to PBS and pre-immune serum. Scale-bars: 100 μm
Anti-rTsSP1.1 serum impeded on larval intrusion of gut mucosa
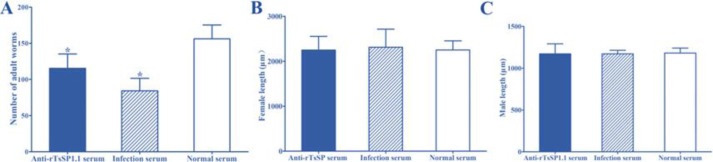
Challenge of mice with the ML pre-incubated with anti-rTsSP1.1 serum revealed a 26.08% reduction of intestinal adult burdens at 5 dpi compared to normal serum group (F = 36.519, P <0.001) (Fig. 7 A). However, the length of female and male adults among three groups of infected mice has no statistically difference (F female= 0.2609, F male = 0.0445, P > 0.05) (Fig. 7B, C). The results suggested that anti-rTsSP1.1 serum impeded on larval intrusion of gut mucosa, as demonstrated by an obvious decline of intestinal adult burdens.
Fig. 7:
Anti-rTsSP1.1 serum impeded on larval intrusion of gut mucosa. A: Intestinal adult worm burden at 5 dpi (n = 10). B: Female lengths (n = 20). C: Male lengths (n = 10). *P < 0.001 compared to pre-immune serum groups
Discussion
Serine protease is an important family of proteolytic enzymes, which play an important role in host invasion, worm growth, anticoagulation, and immune escape. Various serine proteases have been identified from various T. spiralis stages. Moreover, serine protease expression levels at the IIL stage were higher than the ML stage (3). Immune sera against T. spiralis serine proteases (TspSP1.2) partially impeded gut epithelium intrusion by the IIL. Silencing of TsSP1.2 by RNAi suppressed larval intrusion and development (13). Immunization of mice with recombinant serine protease or DNA vaccine showed a partial immune protection after larval challenge infection. The results demonstrated that serine proteases might play a major role for T. spiralis invasion, development and survival in host.
In this study, a novel TsSP1.1 was cloned and expressed. The TsSP1.1 had one functional domain of serine protease family. However, enzyme activity of rTsSP1.1which hydrolyzed the specific substrate was not observed (data not shown). The absence of enzymatic activity of rTsSP1.1 expressed in this study is likely because of incorrect folding of rTsSP1.1 in a prokaryotic expression system. Therefore, to obtain rTsSP1.1 with native enzyme activity, eukaryotic expression system should be used in further study.
On Western blotting analysis, rTsSP1.1 was recognized by anti-rTsSP1.1 serum and infection serum. Several natural TsSP1.1 protein bands of 38.9–76.6 kDa in ML crude antigens and ML ES antigens were identified by anti-rTsSP1.1 serum and infection serum, suggesting that natural TsSP1.1 is a secretory protein. It is likely because TsSP1.1 might have various isoforms, or this protein might be processed by post-translational processing and modification, or because the TsSP1.1 is one member of T. spiralis serine protease super-family which carried with the same antigenic epitopes.
The IIFA results revealed that native TsSP1.1 was expressed at various T. spiralis stages , and it was located primarily at epicuticle and stichosome of T. spiralis ML and IIL, and embryos of females, indicating that TsSP1.1 as a surface and ES protein took part in T. spiralis intrusion of gut epithelium (14). In the in vitro larval intrusion test, an obvious acceleration role of rTsSP1.1 on larval intrusion of IECs was observed, and the acceleration was rTsSP1.1 dose-dependent and could be owing to the specific binding between rTsSP1.1 and IECs (8). Moreover, the ability of the IIL to intrude into IECs was significantly impeded by anti-rTsSP1.1 antibodies, and the inhibitory effect was dose-dependent of anti-rTsSP1.1 antibodies. It is likely due to the formation of an immune complex of TsSP1.1 and anti-TsSP1.1 antibodies at the IIL anterior, which blocked the direct contacts between larval and IECs, subsequently, intercept larval intrusion (9). The results of animal experiment revealed that anti-rTsSP1.1 serum impeded on larval intrusion of gut mucosa, it is likely because anti-rTsSP1.1 antibodies neutralized and blocked the native serine protease present on IIL epicuticle, which impaired serine protease to hydrolyze and destroy the barrier integrity of gut epithelium (15).
Conclusion
TsSP1.1 was a surface and excretion/secretion protein, which was expressed at various T. spiralis stage worms, and it was located primarily at cuticle, stichosome, and embryos of the parasite. rTsSP1.1 accelerated larval intrusion of IECs, whereas anti-rTsSP1.1 antibodies impeded larval intrusion. The acceleration and impeding role was dose-dependently related to the TsSP1.1 and anti-TsSP1.1 antibodies. Block of the IIL with anti-rTsSP1.1 serum also impeded on larval intrusion of gut mucosa. The results indicated that TsSP1.1 participates in T. spiralis intrusion of gut epithelium.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81971952).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Pozio E. World distribution of Trichinella spp. Infections in animals and humans. Vet Parasitol. 2007;149:3–21. [DOI] [PubMed] [Google Scholar]
- 2.Cui J, Jiang P, Liu LN, et al. Survey of Trichinella infections in domestic pigs from northern and eastern Henan, China. Vet Parasitol. 2013;194(2–4):133–5. [DOI] [PubMed] [Google Scholar]
- 3.Liu RD, Cui J, Liu XL, et al. Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Trop. 2015;150:79–86. [DOI] [PubMed] [Google Scholar]
- 4.Wang ZQ, Li LZ, Jiang P, Liu LN, Cui J. Molecular identification and phylogenetic analysis of Trichinella isolates from different provinces in mainland china. Parasitol Res. 2012;110:753–757. [DOI] [PubMed] [Google Scholar]
- 5.Jiang P, Wang ZQ, Cui J, Zhang X. Comparison of artificial digestion and baermann’s methods for detection of Trichinella spiralis pre-encapsulated larvae in muscles with low-level infections. Foodborne Pathog Dis. 2012;9:27–31. [DOI] [PubMed] [Google Scholar]
- 6.Song YY, Zhang Y, Yang DQ, et al. The immune protection induced by a serine protease inhibitor from the foodborne parasite Trichinella spiralis. Front Microbiol. 2018;9:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu LN, Cui J, Zhang X, Wei T, Jiang P, Wang ZQ. Analysis of structures, functions, and epitopes of cysteine protease from Spirometra erinaceieuropaei spargana. Biomed Res Int. 2013;2013:198250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun GG, Song YY, Jiang P, et al. Characterization of a Trichinella spiralis putative serine protease. Study of its potential as sero-diagnostic tool. PLoS Negl Trop Dis. 2018; 12(5):e0006485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang XZ, Sun XY, Bai Y, et al. Protective immunity in mice vaccinated with a novel elastase-1 significantly decreases Trichinella spiralis fecundity and infection. Vet Res. 2020;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Ren HJ, Liu RD, Wang L, Zhang ZF, Wang ZQ. Phage-displayed specific polypeptide antigens induce significant protective immunity against Trichinella spiralis infection in balb/c mice. Vaccine. 2013;31:1171–1177. [DOI] [PubMed] [Google Scholar]
- 11.Hu CX, Zeng J, Hao HN, et al. Biological properties and roles of a Trichinella spiralis inorganic pyrophosphatase in molting and developmental process of intestinal larval stages. Vet Res. 2021; 52(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ManWarren T, Gagliardo L, Geyer J, McVay C, Pearce-Kelling S, Appleton J. Invasion of intestinal epithelia in vitro by the parasitic nematode Trichinella spiralis. Infect Immun. 1997;65:4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang DQ, Zeng J, Sun XY, et al. Trichinella spiralis: Rnai-mediated silencing of serine protease results in reduction of intrusion, development and fecundity. Trop Biomed. 2020;37:932–946. [DOI] [PubMed] [Google Scholar]
- 14.Liu RD, Cui J, Wang L, Long SR, Zhang X, Liu MY, Wang ZQ. Identification of surface proteins of Trichinella spiralis muscle larvae using immunoproteomics. Trop Biomed. 2014;31:579–591. [PubMed] [Google Scholar]
- 15.Li CY, Bai X, Liu XL, et al. Disruption of epithelial barrier of caco-2 cell monolayers by excretory secretory products of Trichinella spiralis might be related to serine protease. Front Microbiol. 2021;12: 634185. [DOI] [PMC free article] [PubMed] [Google Scholar]