Abstract
Sociality and cooperative breeding are associated with enhanced longevity in insects and birds, but whether this is also true for mammals is still subject to debate. African mole-rats (Bathyergidae) have recently been claimed to be the only mammalian family in which such an association may exist because cooperatively breeding bathyergids seem to be substantially longer lived than solitary bathyergids. However, although ample longevity data are available for several social bathyergids, almost nothing is known about mortality distribution and lifespan in solitary bathyergids. Here we present robust long-term data on the longevity of a solitary African mole-rat, the silvery mole-rat Heliophobius argenteocinereus. Our findings show that this species is much longer-lived than previously believed. Nonetheless, our comparative analysis suggests that sociality has indeed a positive effect on longevity in this family. We argue that the extreme longevity seen particularly in social bathyergids is probably caused by a combination of subterranean lifestyle and cooperative breeding.
Keywords: longevity, Heliophobius argenteocinereus, mole-rats, sociality, ageing, subterranean
1. Introduction
African mole-rats (Bathyergidae) are subterranean rodents endemic to sub-Saharan Africa. The family contains solitary as well as social, cooperatively breeding species, predestining it for assessing the influence of sociality on other life-history traits, such as longevity.
Whether cooperative breeding is associated with increased longevity in mammals has been much debated (e.g. [1–3]). Recently, Thorley [4] identified the Bathyergidae as the only mammalian family in which these two traits might be connected, because social bathyergids appear to be longer-lived than solitary bathyergids. However, the author pointed out that because solitary mole-rat species have rarely been kept in captivity, their maximal lifespan may be underestimated, especially when compared to social bathyergids which have been studied for much longer times and in more laboratories (e.g. [5–7]). Therefore, reliable longevity data for solitary mole-rat species are urgently needed.
Obtaining such information is difficult, because solitary subterranean mammals very exceptionally breed in captivity. The available information about ontogeny and longevity in solitary subterranean mammals is therefore scarce and unreliable in almost every reported case.
Here we present the first long-term dataset with a meaningful sample size to quantify the longevity of a solitary subterranean rodent, the silvery mole-rat Heliophobius argenteocinereus. Previous estimates of its maximum lifespan were approximately 7.5 years [8]. We show here that this species is much longer-lived than previously reported. Despite that, our comparative analysis suggests that social bathyergids live longer than solitary bathyergids.
2. Material and methods
(a) . Longevity of silvery mole-rats
Data were collected over ca 20 years (1999–2019) during which silvery mole-rats were kept at the University of South Bohemia, Czech Republic (see supplementary information for details on the study species and husbandry). Based on sex-specific growth curves (electronic supplementary material, figure S1), age at capture could be estimated for all animals with capture weights of less than 175 g; age data from these individuals could therefore be treated as uncensored data points in the Kaplan–Meier analysis.
Animals weighing 175 to 200 g at capture were attributed a minimum age of 1 year (males) or 1.5 years (females), whereas animals with capture weights of more than 200 g were attributed a minimum age of 2 years regardless of sex (electronic supplementary material, figure S1). Data points from all these individuals were right-censored in the Kaplan–Meier analysis because their ages at capture could not be estimated with sufficient accuracy. Kaplan–Meier estimators have been calculated and plotted with GraphPad Prism (5.01).
(b) . Estimating causal effects of sociality on longevity
We compiled longevity data for as many bathyergid species as possible from scientific literature and databases, our own husbandry data, and surveys of scientists and zoological gardens who have kept African mole-rats in the past or at present. We then used maximum recorded lifespan (MLrc) and maximum lifespan residual (MLrs) as outcome variables in the comparative analysis. Using maximum lifespan as the (only) longevity metric in comparative studies has been criticized (e.g. [9]). Yet, for several bathyergid species, it was the only reported metric, while no information about the distribution of ages at death was available. Without this information, it is impossible to compare more robust metrics like, e.g. median longevity or scaled life expectancy. Given that the number of bathyergid species is limited anyway, we, therefore, relied on maximum longevities (and their residuals) in our cross-species comparison. Both outcomes were assumed to come from multivariate normal distributions with a phylogenetic covariance structure among observations (see below).
As for predictors, we created an index variable for sociality by dividing the species into solitary (1) and social (2). We used sample size as another predictor (table 1). As for body mass, we used species averages from the literature or our own data. Prior to analyses, continuous variables were mean-centred and divided by two standard deviations. This allowed for easier assignment of priors and more efficient sampling [18] as well as for appropriate comparison with coefficients of binary predictors, such as sociality (see [19]).
Table 1.
Longevity records of African mole-rats. Species that were not included in the analysis of [4], or values that have been updated compared to [4] and/or [8], are highlighted in bold. MLexp = 4.88M0.153 (see [10]); MLrs = MLrc/MLexp.
| species | body mass (g) | social organization | maximum lifespan recorded MLrc (reference) | maximum lifespan expected (MLexp) | maximum lifespan residual (MLrs) | sample size (references) |
|---|---|---|---|---|---|---|
| Heterocephalus glaber | 45 | social | 37 years Can et al. [11] | 8.7 years | 422% | 3299 Ruby et al. [7] |
| Fukomys mechowii | 378 | social | 26 years Begall et al. [12] | 12.1 years | 215% | 224 Begall et al. [12] |
| Fukomys anselli | 90 | social | 21.5 years (own data P. Dammann) | 9.71 years | 221% | 339 Dammann et al. [13] |
| Fukomys damarensis | 180 | social | 20 years Fang et al. [14] | 10.8 years | 185% | >1000 (N. Bennett personal communication) |
| Fukomys ‘Nsanje’a | 110 | social | 18 years (own data R. Šumbera) | 10.02 years | 180% | 110 (own data R. Šumbera and S. Begall personal communication) |
| Cryptomys hottentotus | 90 | social | 11.75 years (M. Noell (Cincinnati Zoo) personal communication) | 9.71 years | 121% | 13 (N. Bennett personal communication; M. Oosthuizen personal communication; M. Noell (Cincinnati Zoo) personal communication; [15]) |
| Heliophobius argenteocinereus |
200 | solitary | 14.6 years (this study) | 10.97 years | 133% | 101 (this study) |
| Georychus capensis | 181 | solitary | 12.2 years (M. Noell (Cincinnati Zoo) personal communication) | 10.81 years | 113% | 41 (N. Bennett personal communication; M. Noell (Cincinnati Zoo) personal communication; [15]) |
| Bathyergus suillus | 780 | solitary | >6 years Bennett et al. [16] | 13.52 years | >44 | 16 (N. Bennett personal communication; M. Noell (Cincinnati Zoo) personal communication; [15]) |
aProvisionally named after capture locality; molecular data suggest that these animals belong to F. darlingi [17].
To estimate the causal effect of sociality, we first derived a set of assumptions representing the data-generating process, which was summarized in the form of a directed acyclic graph (DAG; see e.g. [20,21]). Given that the DAG is correct, it allows for the estimation of causal effects in observational data by telling which variables to (and not to) control for in order to minimize confounding bias. Based on the assumptions implied by our DAG (electronic supplementary material, figure S2), we then modelled the data in the form of a structural causal model where each variable is assumed to be a function of those variables with a direct arrow pointing at them [22].
Our theoretical estimands, i.e. the quantities of interest, were the total as well as the direct causal effect of sociality (see [22–24]). To estimate the total causal effect of sociality, we controlled for body mass and phylogeny, as implied by our causal graph. Note that there is a link between body mass and sociality in Bathyergidae, with solitary species tending to be larger than social species ([25]; table 1). To account for phylogenetic effects, we used a subtree from the mammal phylogeny by Upham et al. [26]. In our DAG notation: sociality ← body mass → longevity ← sociality; sociality ← phylogeny → longevity ← sociality, where arrows represent (the direction of) causal effects. We did not control for sample size when estimating the total effect, because as far as studies on mole-rats are concerned, sample size is—according to our assumptions—not a confound (i.e. common cause of sociality and longevity), but, in fact, a mediator (i.e. part of the causal effect of sociality on longevity goes through sample size). In a DAG notation: sociality → sample size → longevity (for a full DAG, see electronic supplementary material, figure S2). This is because social species are much better studied than solitary species, resulting in higher sample sizes, which increases the chance of detecting long-lived individuals [4].
To assess the degree of mediation via sample size, we also estimated the direct effect of sociality by additionally controlling for sample size. Causal effects were computed as the contrast between expected longevity of solitary species and expected longevity of social species, averaged over posterior distributions of conditioning variables.
We used a Bayesian framework in which inference is based on posterior distribution of each estimated parameter. Posterior distributions were sampled by running four Hamiltonian Monte Carlo chains, each for 2000 iterations and a 50% warm-up, implemented in a statistical programming language Stan [27]. Posteriors were then summarized with the mean for the central tendency and 89% credible intervals (CI) as a measure of uncertainty (see [18]), as well as by computing the percentage of posterior probability on the expected side of zero (% PP), which can be understood as the amount of support for hypothesized effects. We assigned weakly regularizing priors on all parameters. All models were fitted in RStudio [28], using the rethinking package [18], which uses Stan tools.
3. Results
(a) . Longevity of silvery mole-rats
Survival curves of males and females were nearly indistinguishable (log rank test: π2 = 0.0069; p = 0.934), so sexes were combined henceforth. The resulting full data set consisted of 101 animals with median survival after (estimated) birth of 6.65 years and maximum recorded lifespan of 14.62 years (figure 1). As mean adult body mass is approximately 200 g according to our growth data, this resulted in a maximum lifespan residual (MLrs) of 133% (table 1). Ninety per cent longevity (i.e. the age when 90% of the individuals in our data set had died in) was 14.54 years. Scaled life expectancy (as expressed by dividing median survival by 90% longevity) was 0.457, i.e. individuals died on average at 45.7% of the 90% longevity value.
Figure 1.
Kaplan–Meier curve with 95% confidence intervals (CI) for 101 silvery mole-rats (sexes combined). Black bars indicate right-censored data points.
(b) . Comparative analysis
Longevity data could be collected for nine bathyergid species (table 1).
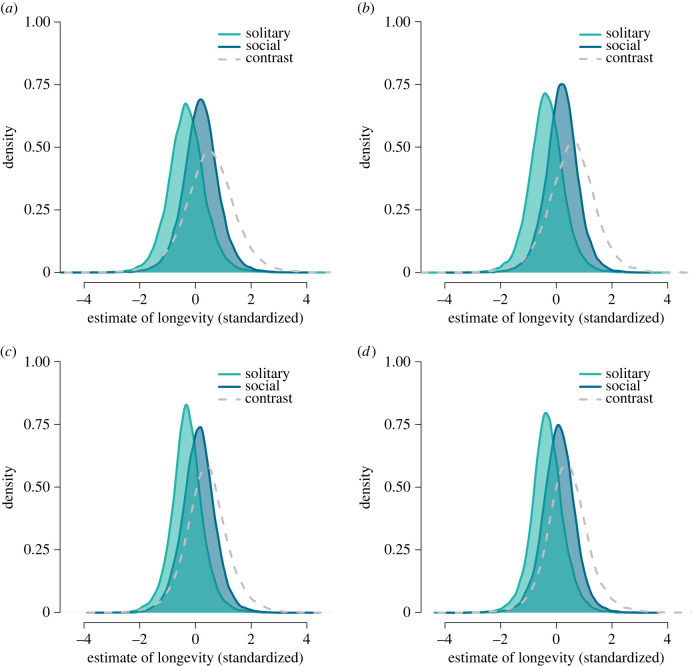
Across these nine species, our analysis revealed a positive (total) effect of sociality on both MLrc (β = 0.52, 89% CI [−0.93, 1.97]; table 2; figure 2a) and MLrs (β = 0.56, 89% CI [−0.83, 1.93]; table 2; figure 2b). That is, expected maximum recorded longevity is on average about 4.75 years higher, and expected maximum lifespan residual is about 59.45% higher for social than for solitary bathyergids. More than 70% of each estimate's posterior probability was positive (table 2).
Table 2.
Summary of causal effects of sociality on maximum recorded lifespan (MLrc) and maximum lifespan residual (MLrs). Estimates of each causal effect, i.e. contrast in expected longevity between social and solitary species, are represented by its mean (β), 89% credible intervals (CI) and the percentage of posterior probability on the expected side of zero (% PP).
| causal effect | Β | 89% CI | % PP |
|---|---|---|---|
| MLrc | |||
| total | 0.52 | −0.93, 1.97 | 73.22 |
| direct | 0.37 | −0.92, 1.69 | 71.54 |
| MLrs | |||
| total | 0.56 | −0.83, 1.93 | 76.05 |
| direct | 0.40 | −0.77, 1.62 | 72.06 |
Figure 2.
Posterior predicted counterfactual distributions of longevity for solitary (turquoise) and social (blue) bathyergids and their contrasts (dashed grey). Each contrast represents the difference in expected longevity between solitary and social species, averaged over the distribution of each counterfactual. Contrasts in plots (a) and (b) show the total effects of sociality, contrasts in plots (c) and (d) show the direct effects of sociality on maximum recorded lifespan (MLrc) and maximum lifespan residual (MLrs), respectively.
When the mediating effect of sample size was taken into account, the (direct) effect of sociality dropped to β = 0.37, 89% CI [−0.92, 1.69] for MLrc and β = 0.40, 89% CI [−0.77, 1.62] for MLrs (table 2; figure 2c,d). In the outcome scales, social species are expected to live approximately 3.78 years longer on average and have an approximately 42.46% higher expected maximum lifespan residual than solitary species. Again, more than 70% of each estimate's posterior probability was positive (table 2).
4. Discussion
This report represents the first longevity study of a solitary bathyergid species, if not of a solitary subterranean mammal species in general, on the grounds of a solid database. We show that silvery mole-rats can live for almost 15 years, i.e. much longer than previously expected. Four of the five oldest individuals in our dataset (including the three individuals with the highest lifespans) provided uncensored data points, which makes us confident that our current estimates of median and maximum lifespan are good approximations of realistic figures for these parameters in H. argenteocinereus.
With a MLrs of 133%, the species exhibits the highest value among all solitary bathyergid species recorded so far, and even a higher value than the social common mole-rat Cryptomys hottentottus. However, the sample for Cryptomys hottentottus is small, and longevity values of species from the social genera Fukomys and Heterocephalus, all of which are based on larger samples, are considerably higher throughout (table 1). Hence, Thorley's [4] assertion that African mole-rats may constitute the only mammalian family in which cooperative breeding is linked to enhanced longevity (although he did not attempt to establish causality) may not be proven wrong even by the updated records for silvery mole-rats. In fact, assuming that our causal assumptions are correct, our comparative analysis supports Thorley's suggestion. Although part of the causal effect of sociality on longevity indeed operates via sample size (table 2; figure 2), the direct effect of sociality on longevity in bathyergids remains positive—and percentages of posterior probabilities remain fairly unchanged—also after accounting for the mediating effect of sample size. Note that the effect of sample size on longevity may well be confounded by an unmeasured variable, which, if accounted for, may have rendered the direct effect of sociality on longevity even higher [24,29–31]. For example, for some species information about sample size and longevity comes from different sources (e.g. laboratories, zoos), which could introduce bias due to specific conditions of respective study environments. Despite that, more than 70% of each estimate's posterior probability was positive, suggesting the effect of sociality on longevity is relatively robust. This said, we acknowledge that given our small sample size (n = 9) and the fact that each contrast accounts for variability in the posterior distribution of model estimates, there is still considerable uncertainty in the magnitude of the reported effects. Therefore, obtaining better data (larger samples, sufficient observation times) especially on the other solitary genera Bathyergus and Georychus remains an important goal.
The high longevity values of silvery mole-rats add additional support for the well-established connection between subterranean lifestyle and enhanced longevity [4,25,32,33]. Taking into account the apparent additive, but probably independent, effect of sociality, the current data suggest that the extraordinary lifespan seen particularly in many social bathyergid mole-rats (Heterocephalus, Fukomys) may be promoted indeed by a combination of their subterranean niche and their social system. Because breeders tend to live longer than non-breeders in social bathyergids [6,7,34], their reported MLrc values are almost always provided by breeding individuals (see, e.g. [5,7,12,35]). Reproductive subdivision of labour is therefore probably key to an understanding of this apparent complementary effect. Several non-mutually exclusive hypotheses may help explain how cooperative breeding promotes longer lifespans in (breeding) mole-rats. For example, survival of breeding individuals could be enhanced due to a load-lightening effect provided by helpers as suggested for cooperatively breeding birds [36]. It should be considered, however, that there is no allo-lactation in mole-rats, hence the (female) breeding mole-rats cannot ‘outsource’ parental care to non-reproductive group members to the same extent as birds. Alternatively, adoption of cooperative breeding may have delayed age at first reproduction—a trait closely related to prolonged lifespan in mammals [37]—in the social mole-rats. However, although the existing data on reproductive parameters in solitary mole-rats are too scarce to allow for final conclusions, there are currently no indications that age at first reproduction differs much between social and solitary mole-rat species. That said, we consider the ‘sheltered-burrow hypothesis' the best explanation for the observed association. Field observations suggest that mole-rat breeders are less active and spend more time inside or close to the nest than non-breeding individuals [38–41]. This tendency resembles the situation found in eusocial insects, in which the reproductive females of many species live in a sheltered nest that is defended against predators by a large workforce—most probably a crucial factor for the enormous longevities seen in many eusocial insect ‘queens’ [42–44]. Breeders in social bathyergid species thus seem to face, on average, lower predation risk than individuals that are forced to patrol the entire tunnel system, as is the case not only for their non-breeding colony mates but also for solitary species that occupy their tunnel systems alone. In summary, the subterranean niche provides shelter for all bathyergid species regardless of sociality, favouring enhanced longevity in all bathyergid species. However, this sheltering effect may be further reinforced for stable subgroups (breeders) that exist only in the social species, thereby enhancing even further the longevity potential of these species.
Acknowledgements
We are grateful to the Genetic Resources and Biotechnology Committee (GRBC), a technical committee of the National Research Council of Malawi (NRCM), and to the Zambian Wildlife Authority (ZAWA) and the Ministry of Tourism, Environment and Natural Resources, Forestry Department, for permission to collect and export ancestors of our breeding stock. We thank Flo Witte, PhD, for medical editing, and Wilbert N. Chitaukali, Chuma Simukonda and Wilbroad Chansa for cooperation, project logistics, and help in obtaining animals in Malawi and Zambia. We also thank the reviewers Jack Thorley and Jean-Michel Gaillard whose comments and suggestions significantly improved this manuscript. Finally, we are obliged to Radka Pešková for the husbandry and weighing of experimental animals, and to Nigel Bennett, Maria Oosthuizen and Mary Noell (Cincinnati Zoo) for sharing information about longevity and sample sizes of various African mole-rat species.
Ethics
All procedures conformed to the legal requirements of Malawi, Zambia and the Czech Republic and to institutional guidelines (allowance no. 43873/2019-MZE-18134).
Data accessibility
The following data and information are provided as electronic supplementary material [45]: raw data on growth and survival of silvery mole-rats; compilation of longevity data of different baythergid species including sample sizes; R code for modelling causal effects; NEXUS file containing phylogenetic information; word file with details on capture, husbandry and analysis of growth of silvery mole-rats, as well as the DAG representing the assumed causal relationships among the study variables.
Authors' contributions
P.D.: conceptualization, formal analysis, writing—original draft, writing—review and editing; G.Š.: formal analysis, methodology, writing—original draft, writing—review and editing; R.Š.: conceptualization, funding acquisition, project administration, resources, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was supported by the Czech Science Foundation project no. 31-20-10222S and the Grant Agency of the University of South Bohemia no. 014/2022/P.
References
- 1.Kamilar JM, Bribiescas RG, Bradley BJ. 2010. Is group size related to longevity in mammals? Biol. Lett. 6, 736-739. ( 10.1098/rsbl.2010.0348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healy K. 2015. Eusociality but not fossoriality drives longevity in small mammals. Proc. Biol. Sci. 282, 20142917. ( 10.1098/rspb.2014.2917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas ER, Keller L. 2019. The co-evolution of longevity and social life. Funct. Ecol. 34, 76-87. ( 10.1111/1365-2435.13445) [DOI] [Google Scholar]
- 4.Thorley J. 2020. The case for extended lifespan in cooperatively breeding mammals: a re-appraisal. PeerJ. 8, e9214. ( 10.7717/peerj.9214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buffenstein R, Jarvis JUM. 2002. The naked mole rat—a new record for the oldest living rodent. Sci. Aging Knowledge Environ. 2002, pe7. ( 10.1126/sageke.2002.21.pe7) [DOI] [PubMed] [Google Scholar]
- 6.Dammann P, Šumbera R, Massmann C, Scherag A, Burda H. 2011. Extended longevity of reproductives appears to be common in Fukomys mole-rats (Rodentia, Bathyergidae). PLoS ONE 6, e18757. ( 10.1371/journal.pone.0018757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruby JG, Smith M, Buffenstein R. 2018. Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age. Elife 7, e31157. ( 10.7554/eLife.31157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacutu R, et al. 2018. Human ageing genomic resources: new and updated databases. Nucleic Acids Res. 46, D1083-D1090. ( 10.1093/nar/gkx1042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronget V, Gaillard JM. 2019. Assessing ageing patterns for comparative analyses of mortality curves: going beyond the use of maximum longevity. Funct. Ecol. 34, 65-75. ( 10.1111/1365-2435.13474) [DOI] [Google Scholar]
- 10.de Magalhães JP, Costa J, Church GM. 2007. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. Biol. Sci. Med. Sci. 62, 149-160. ( 10.1093/gerona/62.2.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Can E, Smith M, Boukens BJ, Coronel R, Bufenstein R, Riegler J. 2022. Naked mole-rats maintain cardiac function and body composition well into their fourth decade of life. GeroScience 44, 731-746. ( 10.1007/s11357-022-00522-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begall S, Nappe R, Hohrenk L, Schmidt TC, Burda H, Sahm A, Szafranski K, Dammann P, Henning Y. 2021. Life expectancy, family constellation and stress in giant mole-rats (Fukomys mechowii). Phil. Trans. R. Soc. B 376, 20200207. ( 10.1098/rstb.2020.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dammann P, et al. 2019. Comment on ‘Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age’. eLife 8, E45415. ( 10.7554/eLife.45415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang X, et al. 2014. Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Rep. 8, 1354-1364. ( 10.1016/j.celrep.2014.07.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigl R. 2005. Longevity of mammals in captivity: from the living collections of the world. Stuttgart, Germany: Kleine Senckenberg-Reihe, Nr. 48. [Google Scholar]
- 16.Bennett NC, Faulkes CG, Hart L, Jarvis JUM. 2009. Bathyergus suillus (Rodentia: Bathyergidae). Mamm. Species 828, 1-7. ( 10.1644/828.1) [DOI] [Google Scholar]
- 17.Šumbera, et al. Submitted. The biology of an isolated Mashona mole-rat population from southern Malawi, with implications for the diversity and biogeography of the genus Fukomys. Org. Divers. Evol. [Google Scholar]
- 18.McElreath R. 2020. Statistical rethinking: a Bayesian course with examples in R and stan. New York, NY: Chapman and Hill/CRC Press. [Google Scholar]
- 19.Gelman A. 2008. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 27, 2865-2873. [DOI] [PubMed] [Google Scholar]
- 20.Pearl J. 1995. Causal diagrams for empirical research. Biometrika 82, 669-688. [Google Scholar]
- 21.Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10, 37-48. [PubMed] [Google Scholar]
- 22.Pearl J, Glymour M, Jewell NP. 2016. Causal inference in statistics: a primer. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 23.Pearl J. 2001. Direct and indirect effects. In Proceedings of the Seventeenth Conference on Uncertainy in Articial Intelligence, pp. 373-392. San Francisco, CA: Morgan Kaufmann. See https://ftp.cs.ucla.edu/pub/stat_ser/R273-U.pdf. [Google Scholar]
- 24.Acharya A, Blackwell M, Sen M. 2016. Explaining causal findings without bias: detecting and assessing direct effects. Am. Political Sci. Rev. 110, 512-529. [Google Scholar]
- 25.Dammann P, Burda H. 2007. Senescence patterns in African mole-rats (Bathyergidae, Rodentia). In Subterranean rodents: news from underground, pp. 251-263. Berlin, Germany: Springer. [Google Scholar]
- 26.Upham NS, Esselstyn JA, Jetz W. 2019. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter B, et al. 2017. Stan: a probabilistic programming language. J. Stat. Softw. 76, 1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RStudio Team. 2020. RStudio: integrated development for R. Boston, MA: RStudio, PBC. http://www.rstudio.com/. [Google Scholar]
- 29.Cole SR, Hernán MA. 2002. Fallibility in estimating direct effects. Int. J. Epidemiol. 31, 163-165. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery JM, Nyhan B, Torres M. 2018. How conditioning on posttreatment variables can ruin your experiment and what to do about it. Am. Political Sci. Rev. 62, 760-775. [Google Scholar]
- 31.Knox D, Lowe W, Mummolo J. 2020. Administrative records mask racially biased policing. Am. Political Sci. Rev. 114, 619-637. [Google Scholar]
- 32.Novikov EA, Burda H. 2013. Ecological and evolutionary preconditions of extended longevity in subterranean rodents. Biol. Bull. Rev. 3, 325-333. ( 10.1134/S2079086413040051) [DOI] [Google Scholar]
- 33.Healy K, et al. 2014. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. Biol. Sci. 281, 20140298. ( 10.1098/rspb.2014.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt CM, Jarvis JUM, Bennett NC. 2013. The long-lived queen: reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis). Afr. Zool. 48, 193-196. ( 10.1080/15627020.2013.11407583) [DOI] [Google Scholar]
- 35.Dammann P, Burda H. 2006. Sexual activity and reproduction delay ageing in a mammal. Curr. Biol. 16, R117-R118. ( 10.1016/j.cub.2006.02.012) [DOI] [PubMed] [Google Scholar]
- 36.Downing PA, Griffin AS, Cornwallis CK. 2021. Hard-working helpers contribute to long breeder lifespans in cooperative birds. Phil. Trans. R. Soc. B 376, 20190742. ( 10.1098/rstb.2019.0742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey P, Zammuto R. 1985. Patterns of mortality and age at first reproduction in natural populations of mammals. Nature 315, 319-320. ( 10.1038/315319a0) [DOI] [PubMed] [Google Scholar]
- 38.Šklíba J, Lövy M, Burda H, Šumbera R. 2016. Variability of space-use patterns in a free living eusocial rodent, Ansell's mole-rat indicates age-based rather than caste polyethism. Sci. Rep. 6, 37497. ( 10.1038/srep37497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lövy M, Šklíba J, Šumbera R. 2013. Spatial and temporal activity patterns of the free-living giant mole-rat (Fukomys mechowii), the largest social bathyergid. PLoS ONE 8, e55357. ( 10.1371/journal.pone.0055357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francioli Y, Thorley J, Finn K, Clutton-Brock T, Zöttl M. 2020. Breeders are less active foragers than non-breeders in wild Damaraland mole-rats. Biol. Lett. 16, 20200475. ( 10.1098/rsbl.2020.0475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zöttl M, Bensch HM, Finn KT, Hart DW, Thorley JB, Bennett NC, Braude S. 2022. Capture order across social bathyergids indicate similarities in division of labour and spatial organisation. Front. Ecol. Evol. 10, 877221. ( 10.3389/fevo.2022.877221) [DOI] [Google Scholar]
- 42.Hölldobler B, Wilson EO. 1990. The ants. Berlin, Germany: Springer. [Google Scholar]
- 43.Carey JR. 2001. Demographic mechanisms for the evolution of long life in social insects. Exp. Gerontol. 36, 713-722. ( 10.1016/s0531-5565(00)00237-0) [DOI] [PubMed] [Google Scholar]
- 44.Jemielity S, Chapuisat M, Parker JD, Keller L. 2005. Long live the queen: studying aging in social insects. Age 27, 241-248. ( 10.1007/s11357-005-2916-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dammann P, Šaffa G, Šumbera R.. 2022. Data from: Longevity of a solitary mole-rat species and its implications for the assumed link between sociality and longevity in African mole-rats (Bathyergidae). Figshare. ( 10.6084/m9.figshare.c.6277193) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dammann P, Šaffa G, Šumbera R.. 2022. Data from: Longevity of a solitary mole-rat species and its implications for the assumed link between sociality and longevity in African mole-rats (Bathyergidae). Figshare. ( 10.6084/m9.figshare.c.6277193) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The following data and information are provided as electronic supplementary material [45]: raw data on growth and survival of silvery mole-rats; compilation of longevity data of different baythergid species including sample sizes; R code for modelling causal effects; NEXUS file containing phylogenetic information; word file with details on capture, husbandry and analysis of growth of silvery mole-rats, as well as the DAG representing the assumed causal relationships among the study variables.