Figure 2.
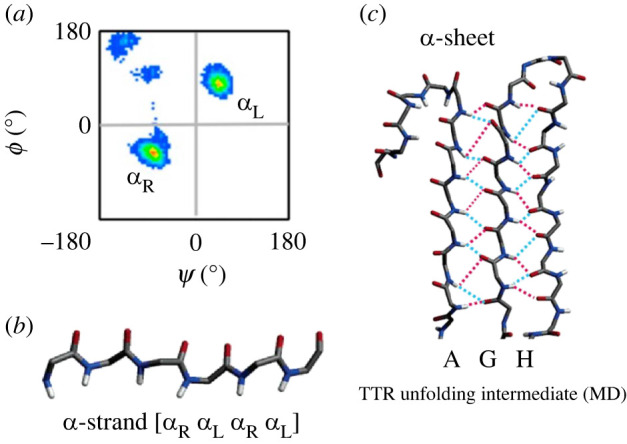
α-sheet structure. (a) Sequential amino acids in the α-strand conformation have backbone Φ and Ψ angles that alternately occupy the αR and αL regions of Ramachandran space. (b) The sequential alternation between αR and αL conformation results in the alignment of carbonyl groups on one side of the peptide backbone, and the alignment of the peptide's amide groups on the other side of the backbone. (c) Bifurcated hydrogen bonding between the A, G and H strands of TTR stabilize the peptide in α-sheet conformation. Reproduced from [12] and [32].
