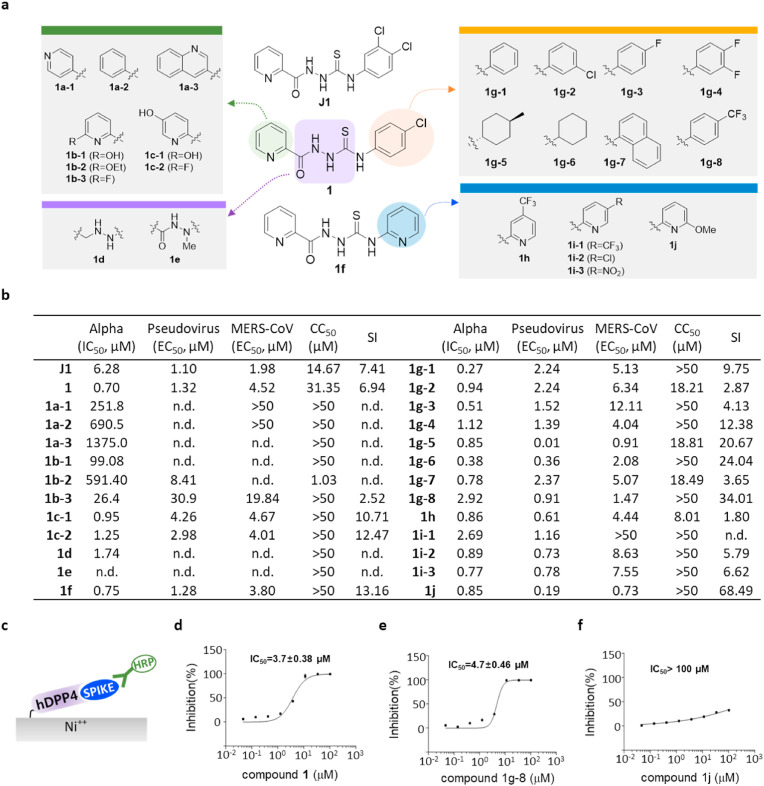
Fig. 2.
Thiosemicarbazide (class I) shows target specificity, and its analogs show regions for their antiviral potency. a) Structure of newly-synthesized thiosemicarbazide derivatives. The modified moieties compared to compound 1 or 1f are highlighted in green (2-pyridine), purple (thiosemicarbazide), orange (pyridine), and blue (p-trifuloromethylphenyl). b) Potency (IC50 or EC50 (μM)), cytotoxicity (CC50 (μM)), and selective index (SI = CC50/EC50 (MERS-CoV infection assay)) of thiosemicarbazide derivatives as evaluated in alpha test, pseudovirus assay, and immunofluorescence-based MERS-CoV infection assay. CC50 values that are >50 μM were considered as 50 μM when calculating SI. n.d; not determined. c) Schematic presentation of the ELISA using hDPP4 and the RBD of the S1 of MERS-CoV spike. d-f) Three compounds, 1 (d), 1g-8 (e), and 1j (f) tested for their inhibiting activity on the binding between hDDP4 and RBD. Each data point represents the mean of triplicate assays with ±SEM, and their IC50 values were calculated through curve fitting analysis using Prism 6.0.