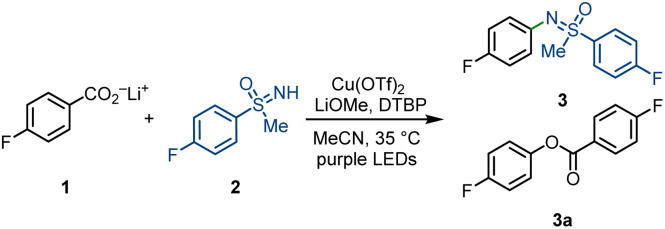
Optimization of the reaction conditionsa.
| ||
|---|---|---|
| Entry | Variation | Yield of 3/3ab (%) |
| 1 | None | 66/8 |
| 2 | H+ instead of Li+ | 44/8 |
| 3 | Na+ instead of Li+ | 60/12 |
| 4 | Li2CO3 instead of LiOMe/DTBP | 48/20 |
| 5 | KF instead of LiOMe/DTBP | 38/40 |
| 6 | 2,6-Difluoropyridine instead of LiOMe/DTBP | 22/36 |
| 7 | Cu(OAc)2 instead of Cu(OTf)2 | 0/0 |
| 8 | DCM instead of MeCN | 0/0c |
| 9 | No DTBP | 0/0 |
| 10 | No LiOMe | 58/8 |
Reaction conditions: 1 (0.05 mmol, 1.0 equiv.), 2 (2.5 equiv.), Cu(OTf)2 (2.5 equiv.), LiOMe (1.0 equiv.), 2,6-di-tert-butylpyridine (DTBP, 2.0 equiv.), MeCN (c = 25 mM), 18 h purple LEDs irradiation, 35 °C.
Yields were determined by 19F NMR using 2-fluorotoluene (2.0 equiv.) as an internal standard.
12% yield of protodecarboxylation product fluorobenzene was observed.
