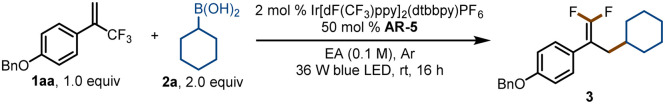
Optimization of conditions for defluorinative alkylation of 1-(benzyloxy)-4-(3,3,3-trifluoroprop-1-en-2-yl)benzene (1aa) with cyclohexylboronic acid (2a)a.
| ||
|---|---|---|
| Entry | Deviation from standard conditions | Yield (%)b |
| 1 | None | 82 (80c) |
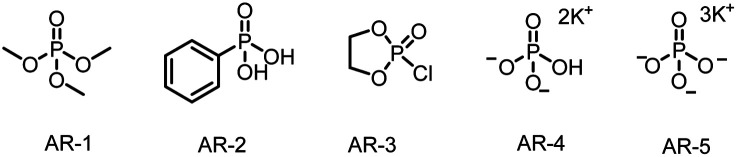
| 2 | AR-4 as the activation reagent | 70 |
| 3 | AR-1–AR-3 as the activation reagent | <50 |
| 4 | 0.2 equiv. of AR-5 | 34 |
| 5 | 1.0 equiv. of AR-5 | 95 |
| 6 | 1.5 equiv. of AR-5 | 94 |
| 7 | 2.0 equiv. of AR-5 | 97 |
| 8 | No photocatalyst | NR |
| 9 | No activation reagent | NR |
| 10 | No light | NR |
| 11 | In air | 32 |
| ||
Standard conditions: 1aa (0.2 mmol), 2a (0.4 mmol), photocatalyst (0.004 mmol), activation reagent (0.1 mmol), ethyl acetate (EA, 2 mL), Ar, 36 W blue LED, rt, 16 h.
Yields were determined by 19F NMR spectroscopy with fluorobenzene as an internal standard. NR = no reaction.
Isolated yield.
