Abstract
Background
Patients with a single ventricle who experience early life growth failure suffer high morbidity and mortality in the perisurgical period. However, long‐term implications of poor infant growth, as well as associations between body mass index (BMI) and outcome in adulthood, remain unclear. We aimed to model BMI trajectories of patients with a single ventricle undergoing a Fontan procedure to determine trajectory‐based differences in baseline characteristics and long‐term clinical outcomes.
Methods and Results
We performed a retrospective analysis of medical records from patients in the Australia and New Zealand Fontan Registry receiving treatment at the Royal Children's Hospital, The Children's Hospital at Westmead, Royal Melbourne Hospital, and Royal Prince Alfred Hospital from 1981 to 2018. BMI trajectories were modeled in 496 patients using latent class growth analysis from 0 to 6 months, 6 to 60 months, and 5 to 16 years. Trajectories were compared regarding long‐term incidence of severe Fontan failure (defined as mortality, heart transplantation, Fontan takedown, or New York Heart Association class III/IV heart failure). Three trajectories were found for male and female subjects at each age group—lower, middle, higher. Subjects in the lower trajectory at 0 to 6 months were more likely to have an atriopulmonary Fontan and experienced increased mortality long term. No association was found between higher BMI trajectory, current BMI, and long‐term outcome.
Conclusions
Poor growth in early life correlates with increased long‐term severe Fontan failure. Delineation of distinct BMI trajectories can be used in larger and older cohorts to find optimal BMI targets for patient outcome.
Keywords: body mass index, congenital heart disease, Fontan procedure, LCGA, single ventricle
Subject Categories: Cardiovascular Surgery, Obesity, Congenital Heart Disease, Heart Failure, Treatment
Nonstandard Abbreviations and Acronyms
- ANZFR
Australia and New Zealand Fontan Registry
- CDC
Centers for Disease Control and Prevention
- FSV
functional single ventricle
- NYHA
New York Heart Association
Clinical Perspective.
What Is New?
This is the first study to model distinct body mass index trajectories in patients with a single ventricle circulation.
Patients with a single ventricle who grow poorly in the first 6 months of life experience greater mortality long term, many years following Fontan procedure.
Although our study found high incidence of overweight and obesity, there was no association found between overweight status and incidence of severe Fontan failure—defined as mortality, heart transplant, takedown, or New York Heart Association grade III/IV heart failure.
What Are the Clinical Implications?
The worldwide prevalence of children and adults with a Fontan circulation, including those achieving overweight and obese status, has been increasing over the past few decades.
Our finding supports early hemodynamic and nutritional interventions in poorly growing infants with a single ventricle circulation in order to improve long‐term outcome.
Future research in this area should use body composition assessments to fully address the associations between body mass index trajectory, body composition, nutritional status, and clinical outcomes in patients with a single ventricle.
Staged surgical palliation of patients born with a functional single ventricle (FSV) may culminate in a Fontan circulation. 1 In the Fontan circulation, systemic output is provided by the FSV and there is passive systemic venous return to the pulmonary arteries. Although survival for patients with a FSV has dramatically improved, patients with a Fontan circulation are still subject to significant early and late morbidity and mortality. 2 , 3
Infants with an FSV commonly experience growth failure, 4 , 5 , 6 , 7 , 8 , 9 which may relate to increased baseline energy demands, reduced nutritional intake, and malabsorption, among other factors. 9 , 10 , 11 , 12 , 13 , 14 , 15 Growth failure in infancy has been shown to correlate with higher perioperative morbidity and mortality for some patients with a FSV. 10 , 12 , 14 , 15 However, the longer term impacts of early life growth failure for patients with a Fontan circulation remain unclear.
In contrast to early life growth failure in patients with a FSV, in older patients, some studies report high rates of overweight and obesity—documented as up to 40% of adult patients with Fontan. 16 , 17 , 18 , 19 , 20 , 21 Potential contributory factors to obesity in patients with a Fontan circulation include disease‐related activity restriction and intensive feeding regimes that aim to reverse early life growth failure. 10 , 12 , 15 , 22 , 23 , 24
Both obesity and underweight status have been associated with adverse perioperative outcomes in patients with congenital heart disease. 14 , 25 , 26 , 27 , 28 Elevated adiposity in adults with a Fontan circulation, when measured by percentage of body fat, has been associated with adverse Fontan‐related outcomes. 29 , 30 , 31 Some studies also suggest an association between higher body mass index (BMI) and poorer performance in surrogate physiological parameters. 32 , 33 , 34 , 35 , 36 However, it is still unclear if overweight and obesity are associated with late Fontan failure.
Distinct BMI trajectories have been identified across the life course in a number of large healthy cohort studies. 37 , 38 , 39 , 40 , 41 Persistently high BMI has been associated with an increased risk of cardiovascular disease and a higher prevalence of cardiovascular risk factors in adulthood. 37 , 40 , 41 , 42 Studies tracking BMI from childhood into adult life in patients with a FSV have been limited by small patient numbers, lack of BMI measurements before Fontan completion, and limited data on the association with late Fontan outcomes. 17 , 36 The effects of changing BMI trajectories on long‐term Fontan outcomes are unknown.
Using data from the ANZFR (Australian and New Zealand Fontan Registry), the primary aims of this study were (1) to determine if discrete BMI trajectories across childhood can be modeled in patients with a FSV and a Fontan circulation and, if so, to evaluate patient characteristics associated with these different trajectories and (2) to examine if BMI trajectories are associated with adverse Fontan‐related outcomes in adulthood. We hypothesized that the extremes of BMI trajectory—that is, persistently high and persistently low—may be associated with adverse Fontan outcomes.
METHODS
Study Population and Design
A retrospective analysis was performed of patients from the ANZFR who underwent Fontan completion at the Royal Children's Hospital, Melbourne or the Children's Hospital at Westmead, Sydney, over the period 1981 to 2018. Data for patients >16 years of age were collected from the ANZFR database and the adult congenital cardiology databases at Royal Prince Alfred Hospital, Sydney or Royal Melbourne Hospital, Melbourne. Only patients with a FSV who survived to Fontan completion are included in the ANZFR. Patients were excluded if the hospital clinical databases did not have sufficient anthropometric data—defined as fewer than 1 observation of height and weight—in any of the age groups: 0 to 6 months, 6 to 60 months, and 5 to 16 years. The project received ethics approval from the Royal Children's Hospital Melbourne Human Research Ethics Committee (Approval Number—36260), and project participants gave informed consent or consent by proxy. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Collection
Patient information was extracted from the ANZFR Redcap electronic research database, hosted by the Murdoch Children's Research Institute, on February 22, 2021. Data gathered included the primary cardiac morphological diagnosis, patient sex, pre‐Fontan clinical data (including number of procedures before Fontan completion), Fontan procedure data (including type of Fontan, conversion, and fenestration), and long‐term outcome data. Participants were assumed to be alive if there was no date of death recorded in the REDCap database (updated annually) or at most recent follow‐up in the hospital medical records (if more recent than the ANZFR update).
Individual paper and electronic patient medical records were used to gather anthropometric data—height and weight—for each patient from birth to 16 years, at which age patients begin to transition to adult care. Multiple height (or length) and weight data points were collected, where available, every month until 6 months of age, and every 6 months thereafter. The following information, relevant to BMI trajectories, was also ascertained: birthweight, gestational age, and major medical comorbidities. Of the potential 532 patients suitable to be included in the study, 496 had sufficient BMI data for analysis. BMI at most recent follow‐up was calculated for all patients included in the analysis. For those patients who were ≥19 years of age at recent follow‐up (n=138), BMI was categorized according to Centers for Disease Control and Prevention (CDC) adult BMI category—underweight (BMI <18.5 kg/m2), normal weight (BMI=18.5–24.9 kg/m2), overweight (BMI=25–29.9 kg/m2), and obese (BMI ≥30 kg/m2). For patients <19 years at most recent follow‐up (n=358), CDC pediatric categories were used: normal weight (BMI <85th percentile), overweight (BMI ≥85th percentile and <95th percentile), and obese (BMI ≥95th percentile). 43
BMI Trajectories and Clinical Outcome
Raw BMI derived from recorded values of height and weight—calculated as weight (kg)/height (m)2—was used to determine trajectory instead of Z scores or percentiles, as raw BMI better maps within‐child variability longitudinally and BMI results are more interpretable and more clinically useful. 44 , 45 Although BMI is not classically used for patients under 2 years of age, 46 we chose to use the same metric across all age groups for the purposes of interage group comparison.
Global BMI curves normally show rapidly increasing BMI from birth to ≈6 months, followed by a decrease to a nadir at ≈5 years, then a steady increase to young adulthood. 47 Because of this nonlinear pattern and to account for changes in BMI after the Fontan procedure, the BMI trajectories were estimated using a fragmented model with 3 time periods. The 3 time periods are bound by the mean age of the BMI trajectory milestones of infancy peak and subsequent adiposity rebound: birth to 6 months (BMI0–6months), 6 months to 5 years (BMI6months–5years), and 5 to 16 years (BMI5–16years). This fragmented approach allowed for more accurate statistical modeling of trajectory patterns given the study sample size, as BMI patterns followed different, distinct shapes in each time period. To account for improved growth post‐Fontan completion, in those where Fontan completion occurred between 4 and 5 years, we chose to include all post‐Fontan BMI measurements to model the 5‐ to 16‐year trajectory. If Fontan completion occurred between 5 and 6 years, then all BMI measurements up until Fontan completion were used in modeling 6‐month to 5‐year trajectory.
BMI trajectory plots were compared with sex‐ and age‐specific BMI percentiles: World Health Organization reference criteria (for patients at 0 to 6 months and 6 to 60 months of age) 47 and CDC reference criteria (for when patients were 5 to 16 years). 43 Reference criteria were chosen by patient age according to CDC and National Health and Medical Research Council guidelines. 48 Using these reference criteria, patients were categorized by trajectory and compared regarding incidence of severe Fontan failure at any point, defined as the combined occurrence of mortality, heart transplantation, Fontan takedown, and New York Heart Association (NYHA) grade III/IV heart failure (dichotomous variable: NYHA III/IV [yes/no]). Association between BMI trajectory and components of severe Fontan failure were also assessed. Baseline characteristics were compared between the 3 trajectories for each age group.
Statistical Analysis
A latent class growth analysis model was used to estimate the trajectories via Mplus version 8.4 (Muthen & Muthen, Los Angeles, CA). 49 , 50 Latent class growth analysis modeling allows for identification of (latent) classes that are defined by their patterns of change over time. The latent class growth analysis estimates mean growth patterns for an age group, rather than estimating individual trajectories for each participant. Individuals are assigned a probability of belonging to each class and then assigned to the class for which they have the highest probability of membership. If a subject did not have any measurements in a specific time period, they were excluded from contributing to the estimation of the mean trajectory and not allocated a class for that age range. If a subject had 1 or more measurements in a specific time period, their information did contribute to the calculation of mean trajectories in that age range using full information maximum likelihood. 51
To determine the optimum number of classes, we assessed model convergence and fit indices (Bayesian information criterion; Akaike's information criterion); entropy, class size, and interpretability. 52 The number of trajectories for each age group was determined by minimizing the Bayesian information criterion; the groups were to be of a reasonable size for statistical analysis with minimal errors in the model—as determined by log‐likelihood. Based on model fit statistics and visualization of expected mean plots, 3 BMI trajectory classes had the greatest discriminatory power at each time period for male and female participants. For all time periods, quadratic trajectories were estimated for each sex separately.
A sensitivity analysis was conducted, comparing the trajectories estimated with the full data set versus trajectories estimated from subjects with 5 or more measurements in each time period. The curves were very similar to those produced that included data from all participants. Therefore, the larger participant cohort was used for modeling and statistical analysis. Two male participants with BMI values far above the overweight population average were removed from trajectory computation for the purpose of classification quality. As 3 distinct trajectories (upper, middle, and lower) could be computed for both male and female subjects across each trajectory age group, the BMI trajectories were combined for male and female subjects in analyzing the association with Fontan‐related outcomes.
Stata version 16.0 (StataCorp, College Station, TX, 2019) was used for all additional statistical analysis. 53 The zanthro package was used for CDC reference BMI percentiles. For comparing baseline characteristics between trajectories, Fisher's exact test and chi‐square tests were used for categorical variables and ANOVA and Kruskal–Wallis tests were used for parametric and nonparametric continuous variables, respectively. Logistic regression was used to assess variables associated with severe Fontan failure. Cox proportional hazards and Kaplan–Meier survival curves were used to assess the risk of mortality associated with BMI trajectory. Years of follow‐up were censored at 16 years to account for low participant numbers beyond this time. For association between BMI trajectory in infancy and adolescence, linear regression was used. Estimates are provided with corresponding 95% CI.
RESULTS
In the full cohort (n=496 total; n=390 at 0 to 6 months, n=463 at 6 to 60 months, and n=458 at 5–16 years), 64% were male, median age at Fontan completion was 4.68 years (interquartile range [IQR] 3.93–5.53), and median age at last follow‐up was 13.57 years (IQR 8.79–18.92). At most recent follow‐up, 358 were in the pediatric age group (≤18 years). Of these, 34 (9.5%) were underweight, 256 (71.5%) were normal weight, 34 (9.5%) were overweight, and 34 (9.5%) were obese. Median BMI percentile for the pediatric patients was 51.10 (IQR 24.30–78.71) (Table S1). In the 138 patients who were ≥19 years of age at last follow‐up, 11 (8%) were underweight, 74 (53.6%) were normal weight, 37 (26.8%) were overweight, and 16 (11.6%) were obese. Median BMI for adults was 24.08 (IQR 21.39, 26.17) (Table S2).
Three distinct BMI trajectories for both sexes could be modeled for each age group (BMI0–6months, BMI6months–5years, and BMI5–16years) classified as lower, middle, and upper (Figure 1, 2, 3 through 1, 2, 3). Subjects in the lower BMI0–6months trajectory had a lower birthweight, a higher number of procedures before Fontan completion, and were more likely to have an atriopulmonary Fontan (Table 1). The baseline characteristics by BMI trajectory group for BMI6months–5years and BMI5–16years are provided in Tables S3 and S4, respectively. For BMI0–6months, the lower trajectory was below the fifth percentile using World Health Organization reference percentile data, whereas for BMI5–16years, the upper trajectory was above the 95th percentile as per CDC reference percentiles.
Figure 1. BMI trajectories for patients with a functional single ventricle (FSV) at 0–0.5‐years of age.
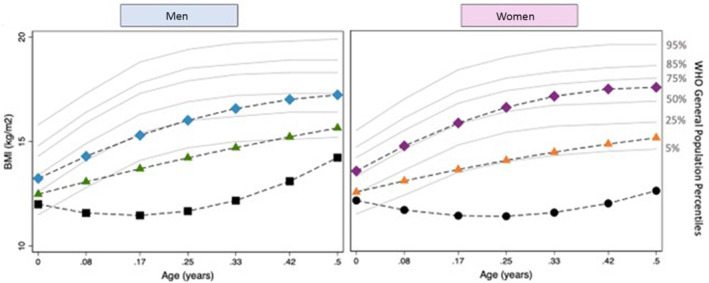
Black=lower trajectory; green/orange=middle trajectory; blue/purple=higher trajectory; gray=World Health Organization (WHO) reference percentiles. BMI indicates body mass index.
Figure 2. BMI trajectories for patients with a functional single ventricle (FSV) at 0.5–5‐years of age.
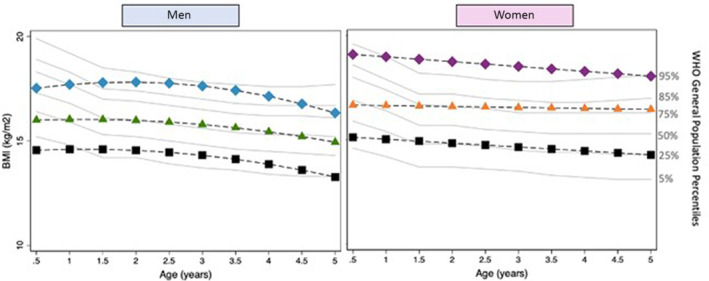
Black=lower trajectory; green/orange=middle trajectory; blue/purple=higher trajectory; gray=World Health Organization (WHO) reference percentiles. BMI indicates body mass index.
Figure 3. BMI trajectories for patients with a functional single ventricle (FSV) at 5–16‐years of age.
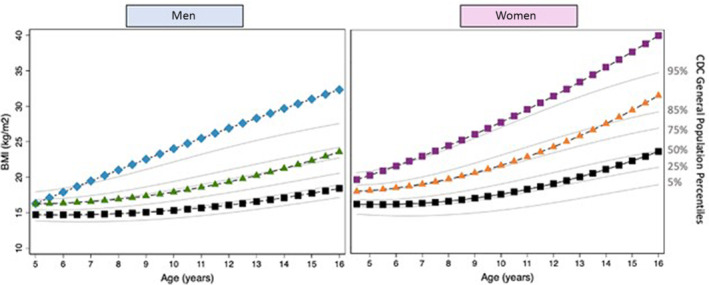
Black=lower trajectory; green/orange=middle trajectory; blue/purple=higher trajectory; gray=Centers for Disease Control and Prevention (CDC) reference percentiles. BMI indicates body mass index.
Table 1.
Baseline Characteristics of BMI0–6months Trajectory Groups (n=390)
| Total (n=390) | Lower (n=31) | Middle (n=255) | Upper (n=99) | P value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 251 (64) | 23 (78) | 160 (62) | 63 (64) | 0.23 |
| Female | 139 (36) | 8 (22) | 95 (37.25) | 36 (36) | |
| Birthweight (kg) (mean±SD) | 3.27±0.62 (n=211) | 2.82±0.64 (n=14) | 3.16±0.56 (n=137) | 3.64±0.56 (n=60) | <0.01 |
| Ventricular morphology | |||||
| Left | 185 (47) | 17 (47) | 125 (49) | 43 (43) | 0.58 |
| Right | 160 (41) | 16 (44) | 102 (40) | 42 (42) | |
| Biventricular | 30 (8) | 1 (3) | 19 (7) | 10 (10) | |
| Indeterminate | 10 (3) | 1 (3) | 5 (2) | 4 (4) | |
| Isomerism/heterotaxy | |||||
| None | 355 (91) | 32 (89) | 229 (90) | 94 (95) | 0.55 |
| Left atrial isomerism | 11 (3) | 1 (3) | 8 (3) | 2 (2) | |
| Right atrial isomerism | 21 (5) | 2 (6) | 16 (6) | 3 (3) | |
| Cardiac position | |||||
| Normal | 352 (90) | 30 (83) | 230 (90) | 92 (93) | 0.43 |
| Dextrocardia/mesocardia | 29 (7) | 5 (14) | 18 (7) | 6 (6) | |
| Primary diagnosis | |||||
| Tricuspid atresia | 63 (16) | 3 (8) | 39 (15) | 21 (21) | 0.15 |
| Double outlet right ventricle | 43 (11) | 3 (8) | 32 (13) | 8 (8) | |
| Double inlet left ventricle | 51 (13) | 6 (17) | 35 (14) | 10 (10) | |
| Pulmonary atresia with ventricular septal defect | 9 (2) | 1 (3) | 7 (3) | 1 (1) | |
| Congenitally corrected transposition of the great arteries | 24 (6) | 1 (3) | 15 (6) | 8 (8) | |
| Ebstein's anomaly | 5 (1) | 0 | 3 (1) | 2 (2) | |
| Unbalanced atrioventricular septal defect | 38 (9) | 5 (14) | 28 (12) | 5 (5) | |
| Pulmonary atresia with intact ventricular septum | 28 (7) | 2 (6) | 22 (9) | 4 (4) | |
| Hypoplastic left heart syndrome | 88 (23) | 7 (19) | 50 (20) | 31 (31) | |
| Other | 39 (10) | 7 (19) | 23 (9) | 9 (9) | |
| Number prior procedures | |||||
| 0–1 | 65 (17) | 5 (14) | 39 (15) | 21 (21) | <0.01 |
| 2 | 200 (51) | 11 (31) | 133 (52) | 56 (57) | |
| 3–6 | 125 (32) | 20 (56) | 83 (33) | 22 (22) | |
| Type Fontan | |||||
| Atriopulmonary | 27 (7) | 6 (17) | 20 (8) | 1 (1) | 0.01 |
| Extracardiac conduit Fontan | 327 (84) | 29 (81) | 208 (82) | 90 (91) | |
| Lateral tunnel Fontan | 32 (8) | 1 (3) | 25 (10) | 6 (6) | |
| Not entered | 4 (1) | 0 | 2 (1) | 2 (2) | |
| Conversion | 11 (3) | 1 (3) | 8 (3) | 2 (2) | 0.90 |
| Fenestration | 142 (40) | 12 (40) | 81 (35) | 49 (52) | 0.02 |
| Center | |||||
| Children's Hospital Westmead | 202 (52) | 17 (47) | 143 (56) | 42 (42) | 0.15 |
| Royal Children's Hospital, Melbourne | 188 (48) | 19 (53) | 112 (44) | 57 (58) | |
| Atrioventricular valve repair/replacement | 46 (12) | 5 (14) | 28 (11) | 13 (13) | 0.73 |
| Date of Fontan (median [IQR]) | 2011 (2002–2015) | 2008 (2000–2015) | 2010 (2001–2014) | 2012 (2006–2015) | 0.18 |
| Age at Fontan (median [IQR]) | 4.60 (3.98–5.38) | 4.81 (4.02–5.46) | 4.62 (3.89–5.45) | 4.49 (4.00–5.23) | 0.41 |
| Age last follow‐up (median [IQR]) | 12.71 (8.33–17.72) | 14.63 (8.25–24.27) | 12.95 (8.32–17.67) | 11.91 (8.33–16.88) | 0.52 |
Values are given as No. (%) unless otherwise indicated. P value X2(2, n=390) indicates comparison between BMI0–6months trajectory groups. IQR indicates interquartile range.
A total of 46 patients experienced severe Fontan failure at a median age of 15 years (IQR 7–26 years) (Table 2). There were 24 deaths occurring at a median age of 21.5 years (IQR 13.5–30.5). Age at last follow‐up was associated with increased risk of heart transplant (P=0.001), NYHA III/IV (P=0.001), and severe Fontan failure (P<0.001). BMI at last follow‐up was not associated with any adverse clinical outcome (Table 3, Tables S1, S2). For the 211 patients with birthweights available, birthweight was not associated with any adverse clinical outcome (Table 3). Factors that were associated with increased incidence of severe Fontan failure include older age at last follow‐up, an atriopulmonary type of Fontan, atrioventricular valve repair or replacement, and 0 to 1 versus ≥2 procedures before Fontan completion (Table 3). Age at last follow‐up was the only variable associated with heart transplant on univariate regression (odds ratio [OR], 1.10, P=0.001). No variables demonstrated a significant association with Fontan takedown and NYHA III/IV on univariate regression.
Table 2.
Clinical End Points for Total Patient Population (n=496)
| End point | No. of events, No. (%) | Age at event, y, median (IQR) | Years post‐Fontan, median (IQR) |
|---|---|---|---|
| Death | 24 (4.8) | 21.5 (13.5–30.5) | 15 (4.0–23.0) |
| Heart transplant | 11 (2.2) | 26 (13.5–31.5) | 16 (3.5–26) |
| Takedown | 3 (0.6) | 4 (3.0–15.0) | 0 (0) |
| New York Heart Association III/IV | 18 (3.6) | 11 (4.0–25.0) | 4.95 (0.8–17.1) |
| Severe Fontan failure | 46 (9.3) | 15 (7.0–26.0) | 8.1 (1.2–20.4) |
IQR indicates interquartile range.
Table 3.
Univariate Logistic Regression for Variables Associated with Mortality or Severe Fontan Failure in the Total Cohort (n=496)
| Mortality OR (95% CI) | P value | Severe Fontan failure OR (95% CI) | P value | |
|---|---|---|---|---|
| Birthweight | 1.62 (0.58–4.65) | 0.35 | 1.35 (0.65–2.78) | 0.42 |
| Age last follow‐up | 1.03 (0.99–1.07) | 0.20 | 1.06 (1.03–1.09) | <0.01 |
| BMI percentile at last follow‐up (pediatric) | 1.00 (0.98–1.02) | 0.90 | 1.00 (0.99–1.01) | 0.85 |
| BMI at last follow‐up (adult) | 1.23 (0.58–2.61) | 0.59 | 1.11 (0.64–1.94) | 0.70 |
| Type of Fontan (relative to atriopulmonary) | ||||
| Extracardiac conduit Fontan | 0.14 (0.05–0.37) | <0.01 | 0.15 (0.07–0.32) | <0.01 |
| Lateral tunnel Fontan | 0.37 (0.10–1.31) | 0.12 | 0.27 (0.09–0.79) | 0.02 |
| Atrioventricular valve Repair/replacement | 1.62 (0.53–4.91) | 0.40 | 2.06 (0.97–4.38) | 0.06 |
| Number of procedures before Fontan (relative to 0–1 prior) | ||||
| 2 | 0.22 (0.09–0.58) | <0.01 | 0.35 (0.17–0.73) | <0.01 |
| 3–6 | 0.27 (0.09–0.78) | 0.02 | 0.49 (0.23–1.05) | 0.07 |
| Ventricular morphology* | 0.87 (0.52–1.47) | 0.61 | 1.45 (0.76–2.74) | 0.25 |
| Isomerism/heterotaxy | 1.04 (0.50–2.19) | 0.91 | 1.07 (0.62–1.84) | 0.80 |
| Cardiac position† | 0.47 (0.06–3.59) | 0.47 | 0.48 (0.11–2.06) | 0.32 |
| Surgical center | 1.38 (0.60–3.14) | 0.45 | 2.09 (0.11–3.92) | 0.21 |
BMI indicates body mass index; and OR, odds ratio.
Right compared with left.
Normal versus dextrocardia/mesocardia.
For BMI0–6months, subjects in the lower trajectory had increased mortality after Fontan completion, adjusting for type of Fontan, number of prior procedures, and atrioventricular valve repair or replacement (Figure 4). This association remained after adjustment for birthweight (compared with lower trajectory—middle: 0.28 [95% CI, 0.10–0.85], P=0.03; higher: 0.17 [95% CI, 0.03–0.91], P=0.04). These deaths occurred at an earlier age in patients in the lowest BMI0–6months trajectory compared with the total population—16 (4–37) versus 20 (3–37) years of age. Different BMI0–6months trajectories were not associated with other outcome measures including severe Fontan failure (Table 4). For BMI6months–5years and BMI5–16years, no significant differences were found in long‐term outcomes based upon trajectory (Tables S5 and S6, respectively).
Figure 4. Kaplan–Meier freedom from mortality by BMI0–6months trajectory.
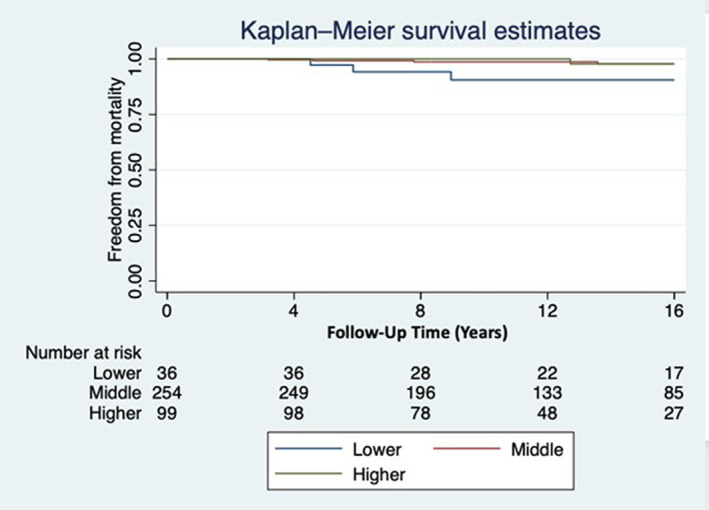
Hazard ratio (compared with lowest trajectory)—Middle: 0.28 (95% CI, 0.10–0.85); P=0.03. Higher: 0.17 (95% CI, 0.03–0.91); P=0.04 [adjusted for type of Fontan, atrioventricular valve repair/replacement, number of prior procedures]. Age at last follow‐up censored at 16 years of age. BMI indicates body mass index.
Table 4.
Long‐Term Clinical Outcome by BMI0–6months Trajectory
| Total (n=390) | Lower (n=31) | Middle (n=255) | Upper (n=99) | P value | |
|---|---|---|---|---|---|
| Severe Fontan failure | 36 (9) | 6 (17) | 24 (9) | 6 (6) | 0.17 |
| Deceased | 18 (6) | 6 (17) | 10 (3.92) | 2 (2) | <0.01 |
| Age deceased, y (median [IQR]) | 20 (10–26) | 16 (6–32) | 24.5 (10–26) | 17.5 (14–21) | 0.90 |
| Age at last follow‐up/censoring, y (median [IQR]) | 15 (8–16) | 13 (8–16) | 12 (8–16) | 13 (8–16) | 0.38 |
| Heart transplant | 8 (2) | 1 (3) | 6 (2) | 1 (1) | 0.69 |
| Takedown | 3 (1) | 1 (3) | 2 (1) | 0 | 0.26 |
| New York Heart Association III/IV | 16 (4) | 1 (3) | 12 (5) | 3 (3) | 0.85 |
| Ventricular failure | |||||
| None‐mild | 184 (91) | 22 (96) | 117 (89) | 45 (94) | 0.84 |
| Moderate | 16 (8) | 1 (4) | 12 (9) | 3 (6) | |
| Severe | 3 (1) | 0 | 3 (2) | 0 | |
| Valvular regurgitation | |||||
| None‐mild | 164 (81) | 19 (83) | 106 (80) | 39 (81) | 0.87 |
| Moderate | 31 (15) | 4 (17) | 19 (14) | 8 (17) | |
| Severe | 8 (4) | 0 | 7 (5) | 1 (2) | |
| Venous thromboembolism | 58 (15) | 6 (17) | 35 (13) | 17 (17) | 0.69 |
Values are given as No. (%) unless otherwise indicated. P value X2(2, N=390) indicates comparison between pediatric BMI0–6months trajectory groups. BMI indicates body mass index.
There was a positive correlation between trajectory at BMI0–6months and that at BMI5–16years (n=357, r s=0.158, P=0.029) (Table S7). Compared with subjects who were in the middle trajectory at both BMI0–6months and BMI5–16years (n=74), those who went from middle/higher to lower (n=180), low to middle/higher (n=9), or low to low (n=24) trajectories did not have a significantly higher risk of mortality (Table 5). None of the patients who were in the highest trajectory at both periods experienced mortality over the study period (0/12 in high BMI0–6months–high BMI5–16years).
Table 5.
Mortality According to Change in BMI Trajectory From BMI0–6months to BMI5–700 16years
| Number | HR | 95% CI | P value | |
|---|---|---|---|---|
| Lower–lower | 24 | 1.75 | 0.32–9.58 | 0.52 |
| Lower–middle/higher | 9 | 4.97 | 0.47–52.08 | 0.18 |
| Middle/high–low | 180 | 0.28 | 0.06–1.40 | 0.12 |
P value indicates comparison between trajectory change groups compared with middle trajectory at both 0–6‐months and 5–16‐years. BMI indicates body mass index; and HR, hazard ratio.
DISCUSSION
In this study, we have made several important findings about BMI and long‐term Fontan‐related outcomes. First, despite high rates of overweight and obesity, particularly in adult patients, we found no significant association between current BMI and Fontan failure. Second, we defined distinct BMI trajectories across childhood in patients with FSV using latent class growth analysis. Third, low BMI trajectory from 0 to 6 months was a risk factor for mortality after Fontan completion independent of birthweight and age at last follow‐up. Fourth, higher BMI trajectories across childhood were not associated with adverse Fontan outcomes. Lastly, patients who changed BMI trajectory between infancy and later childhood—both from low to high, and high to low trajectories—were not at increased risk of mortality, though this finding may be underpowered owing to small sample size.
The strengths of the current study, using data from the ANZFR, include frequent anthropometric measures across childhood both before and after Fontan completion and good ascertainment of long‐term outcomes in a relatively large patient population.
The Long‐Term Impact of Low Weight in Patients With an FSV
We have found that a low BMI trajectory from birth to 6 months is associated with increased mortality after Fontan completion. These deaths in the low trajectory group occurred at a median of 16 (4–37) years of age (as compared with 20 [3–37] years in the total population), many years after Fontan procedure.
Poor growth in early life has been considered a surrogate marker of a poorly functioning circulation. 10 , 12 , 14 , 15 , 23 Although previous reports have demonstrated higher morbidity and mortality in poorly growing patients with an FSV, these studies have been limited to the perioperative period. 10 , 12 , 14 , 15 , 22 , 23 , 24 Our findings demonstrate a mortality risk associated with poor infant growth that persists well into adulthood, independent of baseline differences in characteristics of the BMI trajectory groups including birthweight and type of Fontan. Whether poor infant growth is a cause for or marker of adverse long‐term outcomes remains to be determined. The Pediatric Heart Network trial of enalapril in infants with FSV used weight‐for‐length Z score as a surrogate marker of hemodynamic insufficiency. 54 However, other factors affecting infant growth, such as genetic disorders and lymphatic or gastrointestinal abnormalities, may also affect long‐term outcomes after Fontan completion. 3 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 Nonetheless, our findings highlight the critical importance of tracking early life growth trajectory to predict and potentially later modify adverse Fontan outcomes.
Age‐Related Rise in Prevalence of Overweight and Obesity in Patients With a Fontan Circulation
In patients with a Fontan circulation, there was a higher prevalence in adults than children of both overweight (30% versus 10%) and obesity (14% versus 9%). From age 5 to 16 years, the upper BMI trajectory passed from a normal weight percentile to overweight/obesity. This substantial age‐dependent rise in prevalence of overweight/obesity in patients with Fontan, as in the general population, has been previously reported. 17 , 55 As in populations with noncongenital heart disease, potential modifiable risk factors contributing to this late rise in obesity include reduced physical activity and continued high caloric intake, which may be a legacy from the period pre‐Fontan completion where excess calories are provided in the face of an incompletely palliated circulation. 18 , 21 , 42 , 56 The specific factors contributing to overweight/obesity in patients with a Fontan circulation requires further investigation.
The Association of BMI With Adverse Fontan Outcomes
Various cardiovascular risk factors found to coexist with overweight/obesity—such as hypertension, reduced lung compliance, and diastolic dysfunction—may have a greater effect on the Fontan than the normal biventricular circulation. However, the long‐term impact of overweight and obesity on the Fontan circulation remains uncertain. In an adult population with Fontan (n=79, median age 29.5 years), Martinez et al. (2016) reported a higher incidence of heart failure and increased diuretic requirement in patients who were overweight and obese. 58 These authors also found an increased risk of mortality, heart transplant, or hospice care with increasing BMI (hazard ratio [HR], 3.206 [95% CI, 1.096–9.379] per 1 kg/m2 unit increase in BMI). Decreased transplant‐free survival was also reported in a cohort (n=139, median age=23.2 years) undergoing Fontan conversion. 56 A study by Byrne et al. (2021) (n=104, median age not provided) found that increasing weight gain post‐Fontan—independent of overweight category—was associated with a combined end point of mortality, heart transplant, protein‐losing enteropathy, VO2<50%, and new use of a loop diuretic (HR, 1.36 [95% CI, 1.07–1.73]; P=0.011). 55 By contrast, in a study of 395 adults and children with a Fontan circulation, Chung et al. (2016) found that there was no difference in rates of heart failure comparing the children who had normal weight and children who were overweight/obese. However, adults who were overweight/obese demonstrated lower rates of heart failure than those who were normal and underweight (8% versus 19%; P=0.03). 17 These authors suggested that excess weight gain may be a marker of positive nutritional balance, improved cardiac health, or less severe disease. In our study, we were not able to find an association between current BMI and adverse Fontan‐related outcomes.
BMI as a Measure of Adiposity After Fontan Completion
BMI is an easily derived surrogate measure of body adiposity that incorporates mass differences related to height. However, BMI does not differentiate between lean and fat mass, and several recent studies have observed moderate to severe skeletal muscle mass deficit in patients with a FSV circulation. 29 , 31 , 44 , 45 Thus, in the setting of Fontan‐associated sarcopenia, BMI may underestimate true patient adipose mass. Studies, including ours, using BMI alone may thus misclassify adiposity and its impact after Fontan completion. For example, in a study of adult patients with a Fontan circulation (n=144, age=23±8 years), Cao et al. (2021) found that a 1% increase in percentage of body fat, as measured by dual‐energy X‐ray absorptiometry, was associated with an increased risk of reaching a composite clinical end point of death, heart transplantation, NYHA III/IV heart failure, protein‐losing enteropathy, and/or plastic bronchitis (HR, 1.10 [95% CI, 1.01–1.19]; P=0.03). 29
The Utility of BMI Trajectories in Patients With an FSV Circulation
Modeling of BMI trajectories can capture age of onset, intensity, and duration of exposure to a particular BMI group. In otherwise healthy populations, a high BMI trajectory across childhood has been associated with increased prevalence of adverse cardiometabolic risk factors in young adulthood. 37 , 41 Examination of baseline determinants of BMI trajectories may assist in identification of individuals at increased risk of adverse health outcomes related to either high or low BMI over time. 37
To date, very few studies have examined serial changes of BMI in patients with a FSV reaching Fontan completion. Lambert et al. (2020) studied serial BMIs of 362 patients who were post‐Fontan over a 10‐year period (mean age 21±3.5 years at last follow‐up). 36 For those who were over 20‐years of age, a positive change in BMI was associated with decreased maximal exercise capacity (VO2max) (slope=−1.25, P<0.001).
Chung et al. (2016) reported on 68 patients with serial BMI measurements across childhood. 17 Of the 14/68 overweight/obese adolescents, 8/14 of those were already overweight/obese at ages 2 to 5 years. The low patient numbers and paucity of serial anthropometry allowed neither for modeling of BMI trajectories nor assessment of associations with Fontan outcomes.
Change in BMI Trajectory From Infancy to Later Childhood
We found a correlation between infant and post‐Fontan BMI trajectory. This is in keeping with other studies in congenital heart disease and the general population, showing persistence of growth patterns between childhood and later life. 3 , 8 , 37 , 41 , 58 Early BMI patterns are likely to predict those in later childhood and may be useful for identification of patients who are at increased risk of later excessive weight gain. The number of subjects who changed their BMI trajectory class between infancy and the post‐Fontan period was small, limiting our ability to assess the impact of changes in growth trajectory across time on long‐term Fontan outcomes.
Limitations and Future Research
In this study, the median age at recent follow‐up was 13.6 years with a range of 1 to 47 years. This duration may have been insufficient to have detected other important differences in Fontan outcome based upon BMI or BMI trajectory. Consistent with this was the low number of cases of severe Fontan failure (n=46/496). This cohort and others should be evaluated for late time‐related adverse outcomes. The data examining baseline determinants of BMI trajectories did not incorporate factors beyond those related to the cardiac diagnosis. For example, maternal prepregnancy BMI or diabetes, ethnicity, prematurity, genetic diagnoses, markers of nutritional status, infant feeding practices, physical activity levels, and socioeconomic status, among other factors, were not able to be ascertained. 63 , 64 Prospective birth cohorts of patients with FSV are required to evaluate the late impacts of these factors. As discussed, the use of BMI rather than other measures of body composition may have misclassified body fat status. Future research should incorporate additional metrics of body composition—such as percentage of body fat and skeletal muscle mass—in order to completely assess the adverse implications of excess adiposity in patients with a Fontan circulation.
CONCLUSIONS
We have shown adverse long‐term post‐Fontan outcomes in poorly growing compared with normally growing infants with a FSV circulation. This finding supports early hemodynamic and nutritional intervention in poorly growing infants with a FSV circulation in order to improve long‐term outcome. Distinct BMI trajectories can be modeled using latent class growth analysis. However, neither high BMI nor high BMI trajectory were associated with adverse Fontan outcomes over the duration of our study. Future research in this area should uses body composition assessments to fully address the associations between BMI, body composition, nutritional status, and clinical outcomes in patients with a FSV.
Sources of Funding
The ANZFR gratefully receives funding from the Heart Foundation of Australia, Heart Kids Australia, ANZ Trustees, the Murdoch Children's Research Institute, and the National Health and Medical Research Council. Thomas G Wilson is the recipient of a National Health and Medical Research Council Postgraduate Scholarship (GNT1168270).
Disclosures
Yves d'Udekem is a consultant for Actelion. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025931
For Sources of Funding and Disclosures, see page 11.
References
- 1. Kay WA, Moe T, Suter B, Tennancour A, Chan A, Krasuski RA, Zaidi AN. Long term consequences of the Fontan procedure and how to manage them. Prog Cardiovasc Dis. 2018;61:365–376. doi: 10.1016/j.pcad.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 2. d'Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, Bullock A, Justo RN, Grigg LE, Sholler GF, et al. Redefining expectations of long‐term survival after the fontan procedure: twenty‐five years of follow‐up from the entire population of Australia and New Zealand. Circulation. 2014;130:S32–S38. doi: 10.1161/CIRCULATIONAHA.113.007764 [DOI] [PubMed] [Google Scholar]
- 3. Day RW, Denton DM, Jackson WD. Growth of children with a functionally single ventricle following palliation at moderately increased altitude. Cardiol Young. 2000;10:193–200. doi: 10.1017/S1047951100009100 [DOI] [PubMed] [Google Scholar]
- 4. Ades A, Johnson BA, Berger S. Management of low birth weight infants with congenital heart disease. Clin Perinatol. 2005;32:999–1015, x‐xi, doi: 10.1016/j.clp.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 5. Davis D, Davis S, Cotman K, Worley S, Londrico D, Kenny D, Harrison AM. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d‐transposition of the great arteries. Pediatr Cardiol. 2008;29:328–333. doi: 10.1007/s00246-007-9027-9 [DOI] [PubMed] [Google Scholar]
- 6. Kelleher DK, Laussen P, Teixeira‐Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22:237–244. doi: 10.1016/j.nut.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 7. Rosenthal GL, Wilson PD, Permutt T, Boughman JA, Ferencz C. Birth weight and cardiovascular malformations: a population‐based study. The Baltimore‐Washington infant study. Am J Epidemiol. 1991;133:1273–1281. doi: 10.1093/oxfordjournals.aje.a115839 [DOI] [PubMed] [Google Scholar]
- 8. Schwarz SM, Gewitz MH, See CC, Berezin S, Glassman MS, Medow CM, Fish BC, Newman LJ. Enteral nutrition in infants with congenital heart disease and growth failure. Pediatrics. 1990;86:368–373. doi: 10.1542/peds.86.3.368 [DOI] [PubMed] [Google Scholar]
- 9. Williams RV, Ravishankar C, Zak V, Evans F, Atz AM, Border WL, Levine J, Li JS, Mahony L, Mital S, et al. Birth weight and prematurity in infants with single ventricle physiology: pediatric heart network infant single ventricle trial screened population. Congenit Heart Dis. 2010;5:96–103. doi: 10.1111/j.1747-0803.2009.00369.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlo WF, Carberry KE, Heinle JS, Morales DL, McKenzie ED, Fraser CD Jr, Nelson DP. Interstage attrition between bidirectional Glenn and Fontan palliation in children with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2011;142:511–516. doi: 10.1016/j.jtcvs.2011.01.030 [DOI] [PubMed] [Google Scholar]
- 11. Daymont C, Neal A, Prosnitz A, Cohen MS. Growth in children with congenital heart disease. Pediatrics. 2013;131:e236–e242. doi: 10.1542/peds.2012-1157 [DOI] [PubMed] [Google Scholar]
- 12. Evans CF, Sorkin JD, Abraham DS, Wehman B, Kaushal S, Rosenthal GL. Interstage weight gain is associated with survival after first‐stage single‐ventricle palliation. Ann Thorac Surg. 2017;104:674–680. doi: 10.1016/j.athoracsur.2016.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marino LV, Johnson MJ, Davies NJ, Kidd CS, Fienberg J, Richens T, Bharucha T, Beattie RM, Darlington AE. Improving growth of infants with congenital heart disease using a consensus‐based nutritional pathway. Clin Nutr. 2020;39:2455–2462. doi: 10.1016/j.clnu.2019.10.031 [DOI] [PubMed] [Google Scholar]
- 14. Menon SC, McCandless RT, Mack GK, Lambert LM, McFadden M, Williams RV, Minich LL. Clinical outcomes and resource use for infants with hypoplastic left heart syndrome during bidirectional Glenn: summary from the Joint Council for Congenital Heart Disease National Pediatric Cardiology Quality Improvement Collaborative Registry. Pediatr Cardiol. 2013;34:143–148. doi: 10.1007/s00246-012-0403-8 [DOI] [PubMed] [Google Scholar]
- 15. Srinivasan C, Jaquiss RD, Morrow WR, Frazier EA, Martin D, Imamura M, Sachdeva R. Impact of staged palliation on somatic growth in patients with hypoplastic left heart syndrome. Congenit Heart Dis. 2010;5:546–551. doi: 10.1111/j.1747-0803.2010.00457.x [DOI] [PubMed] [Google Scholar]
- 16. Andonian C, Langer F, Beckmann J, Bischoff G, Ewert P, Freilinger S, Kaemmerer H, Oberhoffer R, Pieper L, Neidenbach RC. Overweight and obesity: an emerging problem in patients with congenital heart disease. Cardiovasc Diagn Ther. 2019;9:S360–S368. doi: 10.21037/cdt.2019.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung ST, Hong B, Patterson L, Petit CJ, Ham JN. High overweight and obesity in Fontan patients: a 20‐year history. Pediatr Cardiol. 2016;37:192–200. doi: 10.1007/s00246-015-1265-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCrindle BW, Williams RV, Mital S, Clark BJ, Russell JL, Klein G, Eisenmann JC. Physical activity levels in children and adolescents are reduced after the fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. 2007;92:509–514. doi: 10.1136/adc.2006.105239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ovroutski S, Ewert P, Alexi‐Meskishvili V, Stiller B, Nurnberg JH, Abdul‐Khaliq H, Hetzer R, Lange PE. Comparison of somatic development and status of conduit after extracardiac Fontan operation in young and older children. Eur J Cardiothorac Surg. 2004;26:1073–1079. doi: 10.1016/j.ejcts.2004.07.021 [DOI] [PubMed] [Google Scholar]
- 20. Pike NA, Evangelista LS, Doering LV, Koniak‐Griffin D, Lewis AB, Child JS. Clinical profile of the adolescent/adult Fontan survivor. Congenit Heart Dis. 2011;6:9–17. doi: 10.1111/j.1747-0803.2010.00475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinto NM, Marino BS, Wernovsky G, de Ferranti SD, Walsh AZ, Laronde M, Hyland K, Dunn SO Jr, Cohen MS. Obesity is a common comorbidity in children with congenital and acquired heart disease. Pediatrics. 2007;120:e1157–e1164. doi: 10.1542/peds.2007-0306 [DOI] [PubMed] [Google Scholar]
- 22. Anderson JB, Iyer SB, Schidlow DN, Williams R, Varadarajan K, Horsley M, Slicker J, Pratt J, King E, Lannon C, et al. Variation in growth of infants with a single ventricle. J Pediatr. 2012;161:16–21e11; quiz 21 e12‐13, doi: 10.1016/j.jpeds.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 23. Burch PT, Gerstenberger E, Ravishankar C, Hehir DA, Davies RR, Colan SD, Sleeper LA, Newburger JW, Clabby ML, Williams IA, et al. Longitudinal assessment of growth in hypoplastic left heart syndrome: results from the single ventricle reconstruction trial. J Am Heart Assoc. 2014;3:e000079. doi: 10.1161/JAHA.114.000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, et al. Pediatric Heart Network Investigators . Contemporary outcomes after the fontan procedure: a pediatric heart network multicenter study. J Am Coll Cardiol. 2008;52:85–98, doi: 10.1016/j.jacc.2008.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Byrne ML, McBride MG, Paridon S, Goldmuntz E. Association of habitual activity and body mass index in survivors of congenital heart surgery: a study of children and adolescents with tetralogy of Fallot, transposition of the great arteries, and Fontan palliation. World J Pediatr Congenit Heart Surg. 2018;9:177–184. doi: 10.1177/2150135117752122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newell AC, Davis K, Wang L, Bichell D, Clay MA. Obesity: risk factor for increased resource utilization at bidirectional Glenn. JPEN J Parenter Enteral Nutr. 2018;42:49–55. doi: 10.1002/jpen.1029 [DOI] [PubMed] [Google Scholar]
- 27. Wallace MC, Jaggers J, Li JS, Jacobs ML, Jacobs JP, Benjamin DK, O'Brien SM, Peterson ED, Smith PB, Pasquali SK. Center variation in patient age and weight at Fontan operation and impact on postoperative outcomes. Ann Thorac Surg. 2011;91:1445–1452. doi: 10.1016/j.athoracsur.2010.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menon SC, Al‐Dulaimi R, McCrindle BW, Goldberg DJ, Sachdeva R, Goldstein BH, Seery T, Uzark KC, Chelliah A, Butts R, et al. Delayed puberty and abnormal anthropometry and its associations with quality of life in young Fontan survivors: a multicenter cross‐sectional study. Congenit Heart Dis. 2018;13:463–469. doi: 10.1111/chd.12597 [DOI] [PubMed] [Google Scholar]
- 29. Cao JY, Tran D, Briody J, Attard C, Hassan EB, Simm P, Burchill L, Twigg SM, Fiatarone‐Singh MA, Ayer J, et al. Impact of adiposity on clinical outcomes in people living with a Fontan circulation. Int J Cardiol. 2021;329:82–88. doi: 10.1016/j.ijcard.2020.12.066 [DOI] [PubMed] [Google Scholar]
- 30. Lubert AM, Alsaied T, Palermo JJ, Anwar N, Urbina EM, Brown NM, Alexander C, Almeneisi H, Wu F, Leventhal AR, et al. Fontan‐associated dyslipidemia. J Am Heart Assoc. 2021;10:e019578. doi: 10.1161/JAHA.120.019578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tran D, D'Ambrosio P, Verrall CE, Attard C, Briody J, D'Souza M, Fiatarone Singh M, Ayer J, d'Udekem Y, Twigg S, et al. Body composition in young adults living with a Fontan circulation: the myopenic profile. J Am Heart Assoc. 2020;9:e015639. doi: 10.1161/JAHA.119.015639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Banks L, McCrindle BW, Russell JL, Longmuir PE. Enhanced physiology for submaximal exercise in children after the Fontan procedure. Med Sci Sports Exerc. 2013;45:615–621. doi: 10.1249/MSS.0b013e31827b0b20 [DOI] [PubMed] [Google Scholar]
- 33. Callegari A, Neidenbach R, Milanesi O, Castaldi B, Christmann M, Ono M, Muller J, Ewert P, Hager A. A restrictive ventilatory pattern is common in patients with univentricular heart after Fontan palliation and associated with a reduced exercise capacity and quality of life. Congenit Heart Dis. 2019;14:147–155. doi: 10.1111/chd.12694 [DOI] [PubMed] [Google Scholar]
- 34. Cohen MS, Zak V, Atz AM, Printz BF, Pinto N, Lambert L, Pemberton V, Li JS, Margossian R, Dunbar‐Masterson C, et al. Anthropometric measures after Fontan procedure: implications for suboptimal functional outcome. Am Heart J. 2010;160:1092–1098, 1098 e1091. doi: 10.1016/j.ahj.2010.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldberg DJ, Zak V, McCrindle BW, Ni H, Gongwer R, Rhodes J, Garofano RP, Kaltman JR, Lambert LM, Mahony L, et al. Exercise capacity and predictors of performance after Fontan: results from the pediatric heart network fontan 3 study. Pediatr Cardiol. 2021;42:158–168. doi: 10.1007/s00246-020-02465-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lambert LM, McCrindle BW, Pemberton VL, Hollenbeck‐Pringle D, Atz AM, Ravishankar C, Campbell MJ, Dunbar‐Masterson C, Uzark K, Rolland M, et al. Longitudinal study of anthropometry in Fontan survivors: pediatric heart network Fontan study. Am Heart J. 2020;224:192–200. doi: 10.1016/j.ahj.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barraclough JY, Garden FL, Toelle BG, Marks GB, Baur LA, Ayer JG, Celermajer DS. Weight gain trajectories from birth to adolescence and cardiometabolic status in adolescence. J Pediatr. 2019;208:89–95 e84, 89, 95.e4, doi: 10.1016/j.jpeds.2018.12.034 [DOI] [PubMed] [Google Scholar]
- 38. Garden FL, Marks GB, Simpson JM, Webb KL. Body mass index (BMI) trajectories from birth to 11.5 years: relation to early life food intake. Nutrients. 2012;4:1382–1398. doi: 10.3390/nu4101382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Movin M, Garden FL, Protudjer JL, Ullemar V, Svensdotter F, Andersson D, Kruse A, Cowell CT, Toelle BG, Marks GB, et al. Impact of childhood asthma on growth trajectories in early adolescence: findings from the Childhood Asthma Prevention Study (CAPS). Respirology. 2017;22:460–465. doi: 10.1111/resp.12928 [DOI] [PubMed] [Google Scholar]
- 40. Oluwagbemigun K, Buyken AE, Alexy U, Schmid M, Herder C, Nothlings U. Developmental trajectories of body mass index from childhood into late adolescence and subsequent late adolescence‐young adulthood cardiometabolic risk markers. Cardiovasc Diabetol. 2019;18:9. doi: 10.1186/s12933-019-0813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Slining MM, Herring AH, Popkin BM, Mayer‐Davis EJ, Adair LS. Infant BMI trajectories are associated with young adult body composition. J Dev Orig Health Dis. 2013;4:56–68. doi: 10.1017/S2040174412000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wellnitz K, Harris IS, Sapru A, Fineman JR, Radman M. Longitudinal development of obesity in the post‐Fontan population. Eur J Clin Nutr. 2015;69:1105–1108. doi: 10.1038/ejcn.2015.68 [DOI] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention . Growth charts ‐ clinical growth charts. 2017.
- 44. Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol. 2007;17:44–50. doi: 10.1016/j.annepidem.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 45. Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z‐score or BMI centile? Eur J Clin Nutr. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090 [DOI] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention . Using the WHO growth charts | growth birth to 2 years. 2007.
- 47. World Health Organization . World Health Organization. Body mass index‐for‐age (BMI‐for‐age). 2007.
- 48. National Health and Medical Research Council . Australian dietary guidelines: providing the scientific evidence for healthier Australian guidelines. National Health and Medical Research Council. 2013.
- 49. Muthén LK, Muthén BO. Mplus statistical analysis with latent variables user's guide. 1998.
- 50. Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. 2007.
- 51. Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6:330–351. doi: 10.1037/1082-989X.6.4.330 [DOI] [PubMed] [Google Scholar]
- 52. Nylund K, Asparouhov T, Muthén B. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. doi: 10.1080/10705510701575396 [DOI] [Google Scholar]
- 53. StataCorp . Stata statistical software: Release 17. 2021.
- 54. Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Byrne RD, Weingarten AJ, Clark DE, Healan SJ, Richardson TL, Huang S, Menachem JN, Frischhertz BP. Sizing up Fontan failure: association with increasing weight in adulthood. Pediatr Cardiol. 2021;42:1425–1432. doi: 10.1007/s00246-021-02628-8 [DOI] [PubMed] [Google Scholar]
- 56. Freud LR, Webster G, Costello JM, Tsao S, Rychlik K, Backer CL, Deal BJ. Growth and obesity among older single ventricle patients presenting for Fontan conversion. World J Pediatr Congenit Heart Surg. 2015;6:514–520. doi: 10.1177/2150135115598212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kogon BE, Ramaswamy V, Todd K, Plattner C, Kirshbom PM, Kanter KR, Simsic J. Feeding difficulty in newborns following congenital heart surgery. Congenit Heart Dis. 2007;2:332–337. doi: 10.1111/j.1747-0803.2007.00121.x [DOI] [PubMed] [Google Scholar]
- 58. Martinez SC, Byku M, Novak EL, Cedars AM, Eghtesady P, Ludbrook PA, Billadello JJ. Increased body mass index is associated with congestive heart failure and mortality in adult Fontan patients. Congenit Heart Dis. 2016;11:71–79. doi: 10.1111/chd.12296 [DOI] [PubMed] [Google Scholar]
- 59. Ono M, Boethig D, Goerler H, Lange M, Westhoff‐Bleck M, Breymann T. Somatic development long after the Fontan operation: factors influencing catch‐up growth. J Thorac Cardiovasc Surg. 2007;134:1199–1206. doi: 10.1016/j.jtcvs.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 60. Anderson JB, Beekman RH, III , Eghtesady P, Kalkwarf HJ, Uzark K, Kehl JE, Marino BS. Predictors of poor weight gain in infants with a single ventricle. J Pediatr. 2010;157:407–413, 413 e401, 413.e1, doi: 10.1016/j.jpeds.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 61. Francois K, Bove T, Panzer J, De Groote K, Vandekerckhove K, De Wilde H, De Wolf D. Univentricular heart and Fontan staging: analysis of factors impacting on body growth. Eur J Cardiothorac Surg. 2012;41:e139–e145. doi: 10.1093/ejcts/ezs194 [DOI] [PubMed] [Google Scholar]
- 62. Power A, Schultz L, Dennis K, Rizzuto S, Hollander AM, Rosenthal DN, Almond CS, Hollander SA. Growth stunting in single ventricle patients after heart transplantation. Pediatr Transplant. 2020;24:e13634. doi: 10.1111/petr.13634 [DOI] [PubMed] [Google Scholar]
- 63. Andrasfay T, Goldman N. Intergenerational change in birthweight: effects of foreign‐born status and race/ethnicity. Epidemiology. 2020;31:649–658. doi: 10.1097/EDE.0000000000001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lim JU, Lee JH, Kim JS, Hwang YI, Kim TH, Lim SY, Yoo KH, Jung KS, Kim YK, Rhee CK. Comparison of World Health Organization and Asia‐Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2465–2475. doi: 10.2147/COPD.S141295 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


