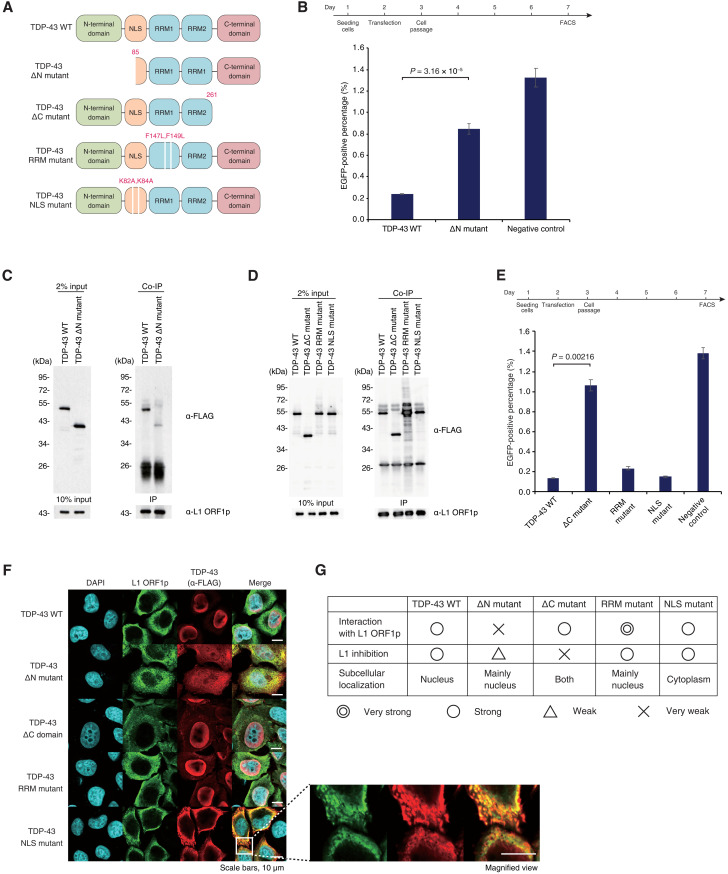
Fig. 5. Interaction with L1 ORF1p is required for TDP-43–mediated L1 retrotransposition.
(A) Illustration of TDP-43 mutants used in this study. (B) The FACS-based retrotransposition assay (see Fig. 2C) showed that retrotransposition frequency was higher in HEK293T cells with ectopic expression of the TDP-43 ΔN mutant compared to the full-length TDP-43. The experimental time course is shown in the top panel. (C) The interaction between the FLAG-tagged TDP-43 ΔN mutant and L1 ORF1p was examined by co-IP of L1 ORF1p in HEK293T cells. The interaction between TDP-43 ΔN mutant and L1 ORF1p was compromised relative to wild-type TDP-43. (D) The interaction between FLAG-tagged TDP-43 mutants (A) and L1 ORF1p was examined by co-IP of L1 ORF1p in HEK293T cells. Loss of either the C-terminal domain or mutation of the NLS did not affect TDP-43’s interaction with L1 ORF1p. (E) L1 retrotransposition frequency in HEK293T cells overexpressing TDP-43 mutants. The experimental time course is shown above. Inhibition of retrotransposition by TDP-43 was compromised by loss of the C-terminal domain but not other mutations. (F) Subcellular localization of L1 ORF1p and TDP-43 mutants in HeLa cells by immunofluorescence staining. The TDP-43 NLS mutant was localized to the cytoplasm, with significant overlap with L1 ORF1p. (G) Summary table of the characteristics of TDP-43 mutants.