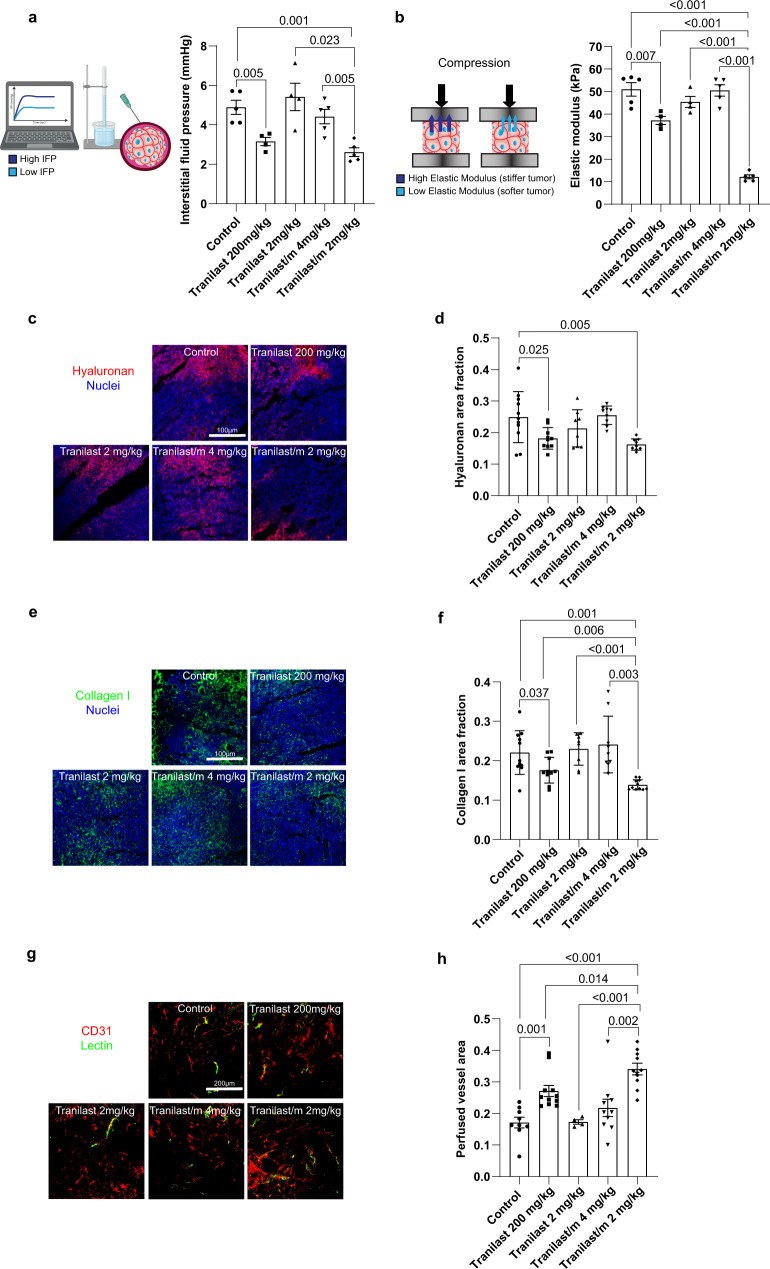
Fig. 2. Normalization effects of free tranilast and Tranilast/m on the TME.
a Quantification of interstitial fluid pressure (IFP) using the wick-in-needle technique in E0771 tumors treated with free tranilast 200 mg/kg (daily, orally), free tranilast 2 mg/kg (daily, i.v.), Tranilast/m 4 mg/kg (every other day, i.v.) and Tranilast/m 2 mg/kg (daily, i.v.) (n = 4–5 mice). b Ex vivo elastic modulus quantification of E0771 tumor specimens (3 × 2 × 2 mm, from the tumor interior) following unconfined compression to a final strain of 30% with a strain rate of 0.1 mm/min (n = 4–5 mice). c Representative fluorescence images of tumor tissue sections stained with anti-hyaluronan (red) antibody and DAPI (blue). Scale bar 100 μm. d Quantification of the area fraction positive for hyaluronan staining treated as indicated (n = 3 mice, N = 3–4 image fields). e Representative fluorescence images of tumor tissue sections stained with anti-collagen I (green) antibody and DAPI (blue). Scale bar 100 μm. f Quantification of the area fraction positive for collagen I staining treated as indicated (n = 3 mice, N = 3–4 image fields). g Immunofluorescence staining of E0771 tumor tissues using the endothelial cell marker anti-CD31 (red) and biotinylated lectin (green) as a measure of vascular perfusion indicated by the colocalization of CD31 and lectin protein (yellow). Scale bar 200 μm. h Perfused vessel fraction in the different treatment groups normalized to total CD31 positive staining (n = 3 mice, N = 3–4 image fields). Data are presented as mean ± SE. For (a, b) (right panels), (d, f, h) statistical analyses were performed by comparing means between two independent groups using the unpaired parametric Welch t test. (a, b) were created with BioRender.com.