Figure 8.
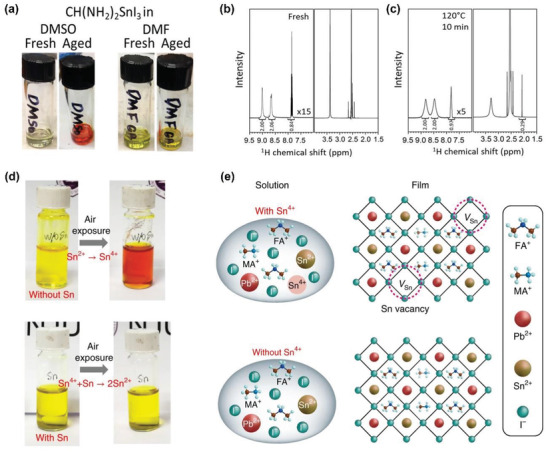
Sn oxidation in THP precursors. a) Images of fresh and aged FASnI3 solutions in DMSO solvent (left) and in DMF solvent (right). 1H NMR spectra of FASnI3 solutions in DMSO solvent b) before and c) after thermal treatment (120°C, 10 min). The peak at 2.07 ppm indicates the presence of DMS. a–c) Reproduced with permission.[ 110 ] Copyright 2020, American Chemical Society. d) Image of Sn oxidation of Sn2+ to Sn4+ in THP solution in ambient air (top), and reduction of Sn4+ to Sn2+ with the addition of metallic Sn powder. e) Schematic representation of Sn vacancy formation due to presence of Sn4+ in mixed Sn‐Pb perovskite precursor solution (top) and suppression of Sn vacancy formation in Sn‐reduced precursor solution with the absence of Sn4+. (d,e) Reproduced with permission.[ 128 ] Copyright 2019, Nature Publishing Group.
