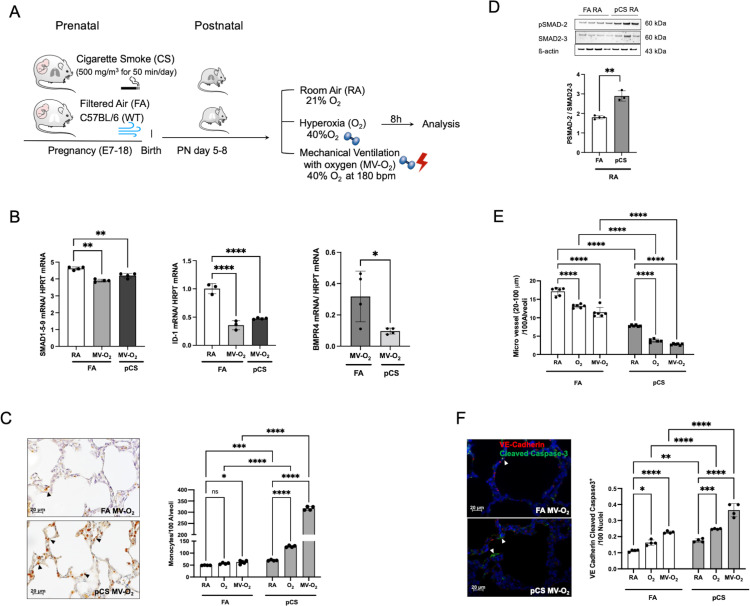
Figure 3.
Early decrease in BMPR2 signalling in early lung vascular injury provoked by exposure to pCS. (A) In a preclinical BPD mouse model, neonatal mice were subjected to non-growth restricting doses of prenatal pCS followed by postnatal MV and/or O2 (FiO2=0.4). (B) Smad 1-5-9 (left panel) and ID1 (middle panel) mRNA expression show a comparable reduction after MV-O2 exposure in pCS and FA mice, whereas BMP4 is further decreased in neonatal mice exposed to pCS followed by MV-O2 as compared with FA controls (right panel). (C) Representative images of IHC stainings indicate increased presence of pulmonary monocytes/macrophages (F4/80) (4 µM, ×400, left panel) following pCS, aggravated by postnatal exposure to MV and/or O2 (right panel), and resulting (D) in a significant increase of pulmonary TGF-ß activity, that is, lung pSMAD2 protein expression. (E) Histological analysis reveals a significant decrease in microvessel number (20–100 µm diameter) induced by pCS in the lungs of neonatal mice when compared with FA controls. (F) Representative images of if staining for cleaved caspase-3 (green) and VE-cadherin (4 µM, ×400, left panel; nuclei (DAPI, blue) in lungs of 5–8 days old pups show significantly enhanced lung EC apoptosis in pCS exposed pups, aggravated byMV and O2 exposure (right panel). Data are mean±SD *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n=3–6 mice/group. quantification of if images in 10 FOV per section in two sections per animal, normalisation of positive cells to 100 nuclei; arrows point to positive cells. ISH quantification in 212.55 µm x 212.55 µm FOV. BMPR2, bone morphogenetic protein receptor 2; BPD, bronchopulmonary dysplasia; EC, endothelial cell; FA, filtered air; FOV, fields of view; pCS, prenatal cigarette smoke.