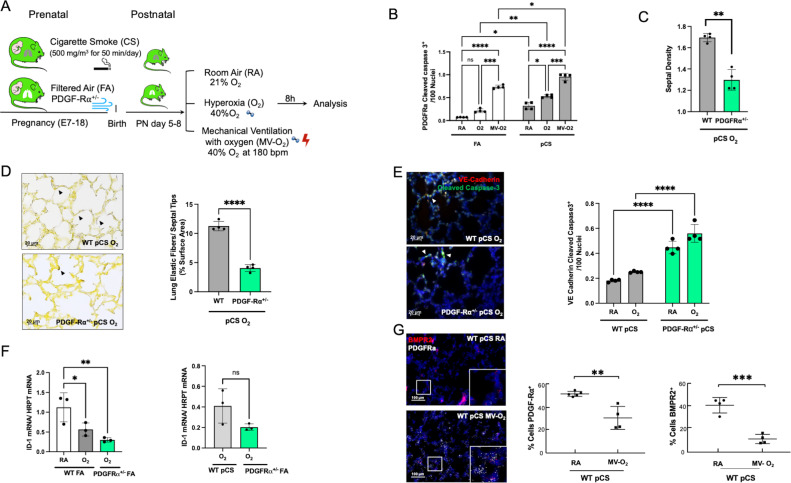
Figure 4.
Aggravated loss of BMPR2 signalling and increased vascular pathology in the presence of BPD characteristic PDGF-Rα deficiency. (A) In our preclinical model, PDGF-Rα heterozygote (PDGF-Rα+/-) neonatal mice and WT littermates were exposed to pCS and postnatal MV and/or hyperoxia (FiO2=0.4) for 8 hours. (B) Exposure to pCS resulted in a significant increase in apoptosis of PDGF-Rα expressing cells (PDGF-Rα and cleaved caspase-3 double positive cells), aggravated by postnatal exposure to O2 or MV-O2. (C, D). The reduction in PDGFR expression is accompanied by an increased loss of septal crests and elastic fibres (Hart’s stain) on postnatal O2 exposure (4 µM, 400X) in the lungs of 5–8 days old mice. (E) Quantification of if staining in lung tissue sections show enhanced EC apoptosis in PDGF-Rα+/- neonatal mice exposed to pCS and postnatal O2, that is, increase in VE-cadherin (red) and cleaved caspase-3 (green) double positive cells (4 µM, ×400, left panel; nuclei (DAPI, blue). (F) Subsequently, mRNA analysis demonstrates decreased ID1 mRNA expression in lungs from PDGF-Rα+/- mice exposed to short-term postnatal O2, exceeding the effect in WT littermates achieved by O2 alone or in combination with pCS. (G) ISH quantification demonstrates the parallel decrease in PDGF-Rα and BMRPR2 transcription in WT mice undergoing prenatal and postnatal injury, that is, pCS and MV-O2. Data are mean±SD. *P<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n=3-4 mice/group. Quantitative analysis of IF images are performed in 10 fields of view (FOV) per section in a total of 2 sections per animal, normalisation of positive cells to 100 nuclei; arrows point to positive cells. ISH quantification in 212.55 µm x 212.55 µm. BMRPR2, bone morphogenetic protein receptor 2; BPD, bronchopulmonary dysplasia; EC, endothelial cell; FA, filtered air; MV, mechanical ventilation; PCS, prenatal cigarette smoke; WT, wild type.