Compounds 4a–e and 6a–ca.
| Entry | Diamine | Product | Time (min) | Yield (%) | Mp (°C) |
|---|---|---|---|---|---|
| 1 |

|
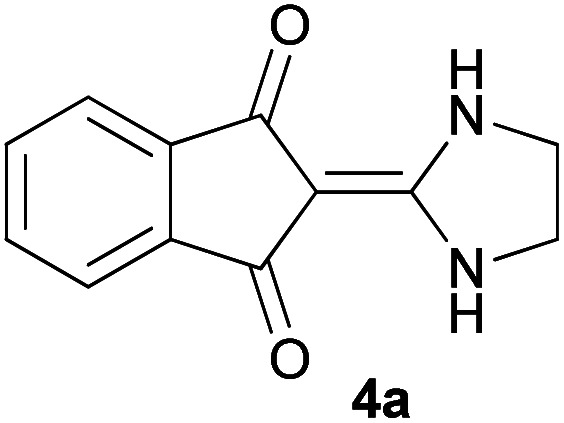
|
10 | 98 | 227–229 |
| 2 |

|
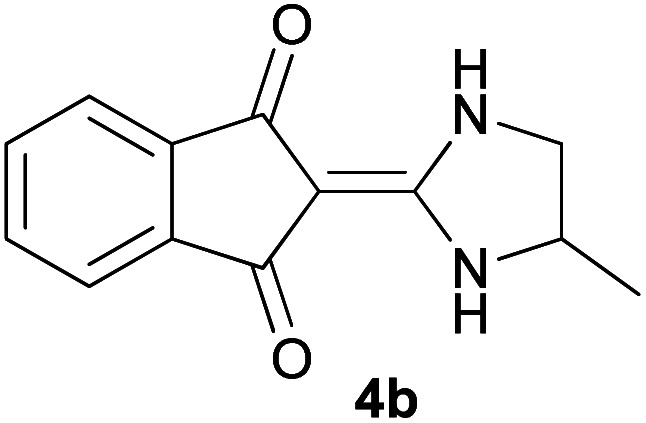
|
25 | 76 | 240–242 |
| 3 |

|
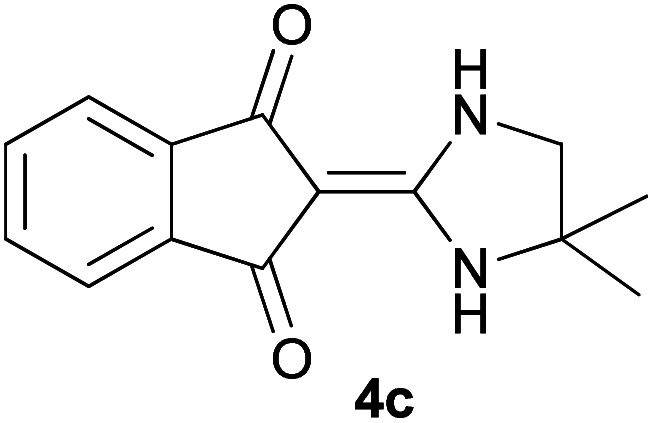
|
30 | 85 | 280–282 |
| 4 |

|

|
15 | 80 | 231–233 |
| 5 |
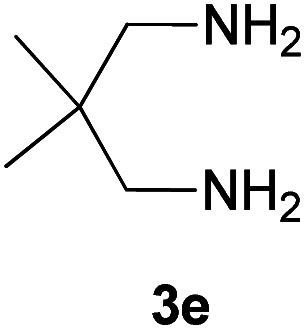
|
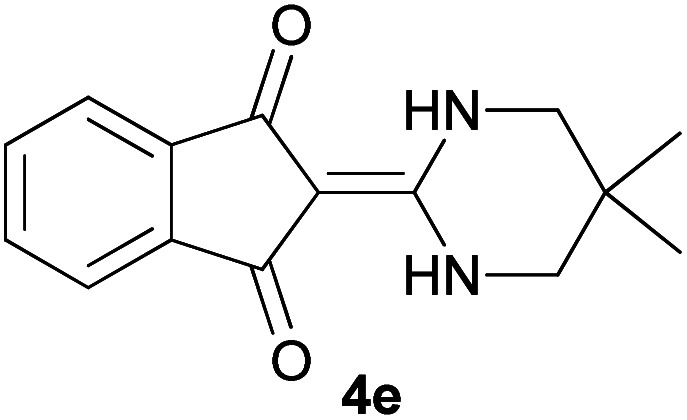
|
20 | 73 | 278–280 |
| 6 |

|

|
120 | 84 | 314–317 |
| 7 |

|

|
90 | 82 | 241–243 |
| 8 |
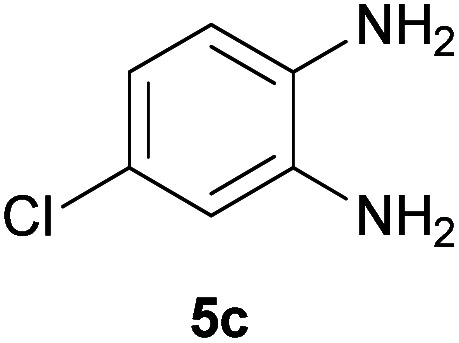
|

|
100 | 78 | 185–188 |
The reactions were performed using ninhydrin (1 mmol), malononitrile (1 mmol), diamine (1 mmol), H2O (10 ml).
