Figure 2 |. Schematic of a muscle cell and the proteins linked to the sarcomere Figure 2. Sarcomere structure in skeletal muscle.
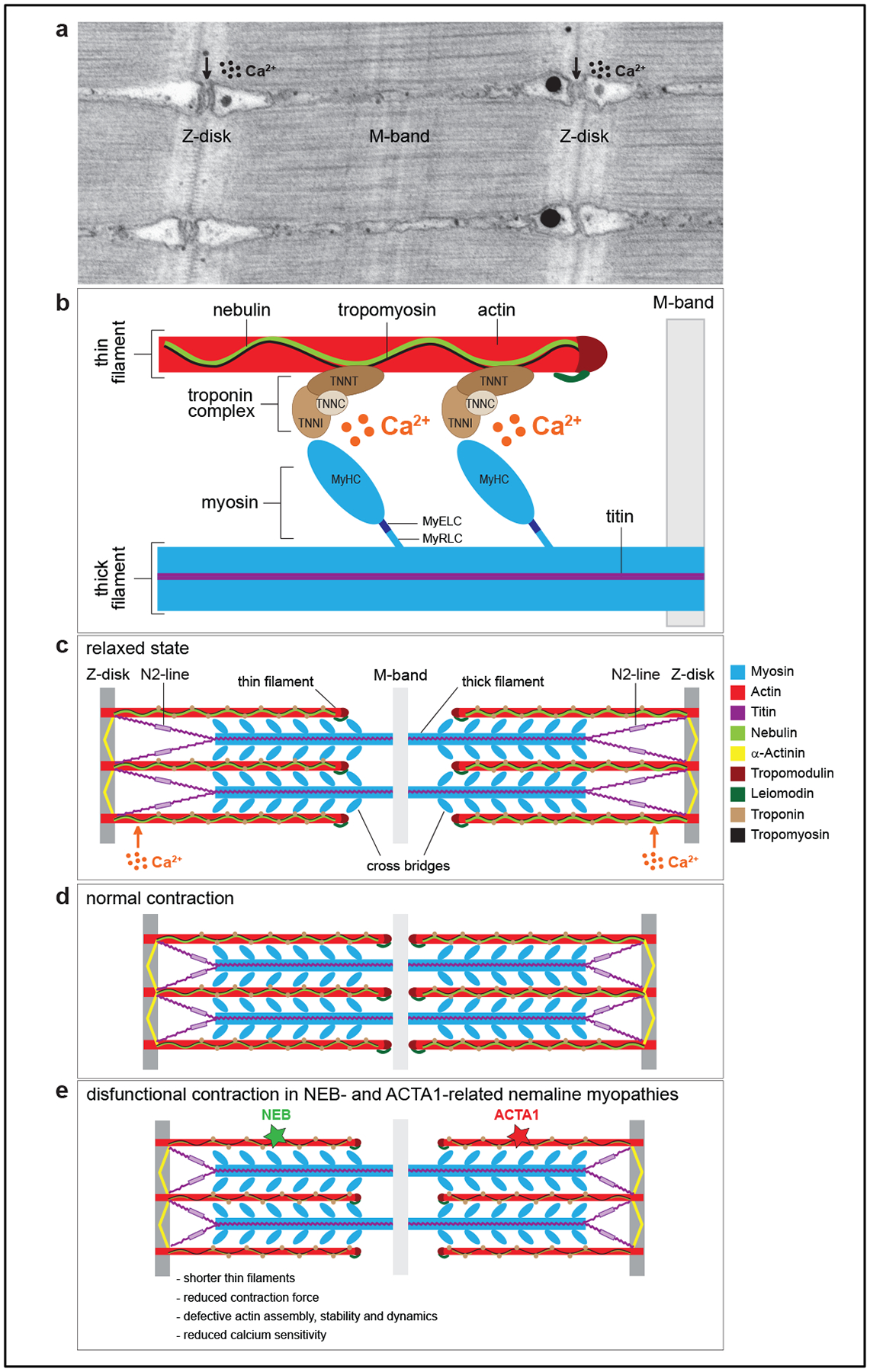
a. Transmission electron micrograph of a longitudinal section of the sarcomere of zebrafish skeletal muscle. Z-disks are visible as electron-dense zig-zag vertical lines, M-band as a smooth dark line in the middle of the sarcomere, and the horizontal striations represent thick and thin filaments. The vacuolated areas are the triads, where excitation contraction coupling takes place. b. Schematic of the major protein components of the sarcomere. Thin filaments are composed of actin, nebulin, tropomyosin and the troponin complex with the following subunits: troponin T (TNNT, binds tropomyosin), troponin I (TNNI, binds actin), and troponin C (TNNC, binds the calcium ions). Thick filaments are composed of myosin and titin. Myosin consists of several domains: a head [two identical myosin heavy chains (MyHC), which bind actin], a neck [one pair of essential light chains (MyELC) and one pair of regulatory light chains (MyRLC)], and a tail. c. Schematic of a sarcomere in a relaxed state. d. Schematic of a sarcomere in contracted state. e. Schematic of a sarcomere during a defective contraction, which is a hallmark of thin-filament related nemaline myopathies. Mutations in nebulin (NEB) and actin (ACTA1) can lead to shorter thin filaments, defective actin assembly and dynamics, reduced force during muscle contraction, and lower sensitivity to calcium ions.
