Figure 4 |. The triad is a muscle-specific substructure that is critical for mediating excitation contraction coupling.
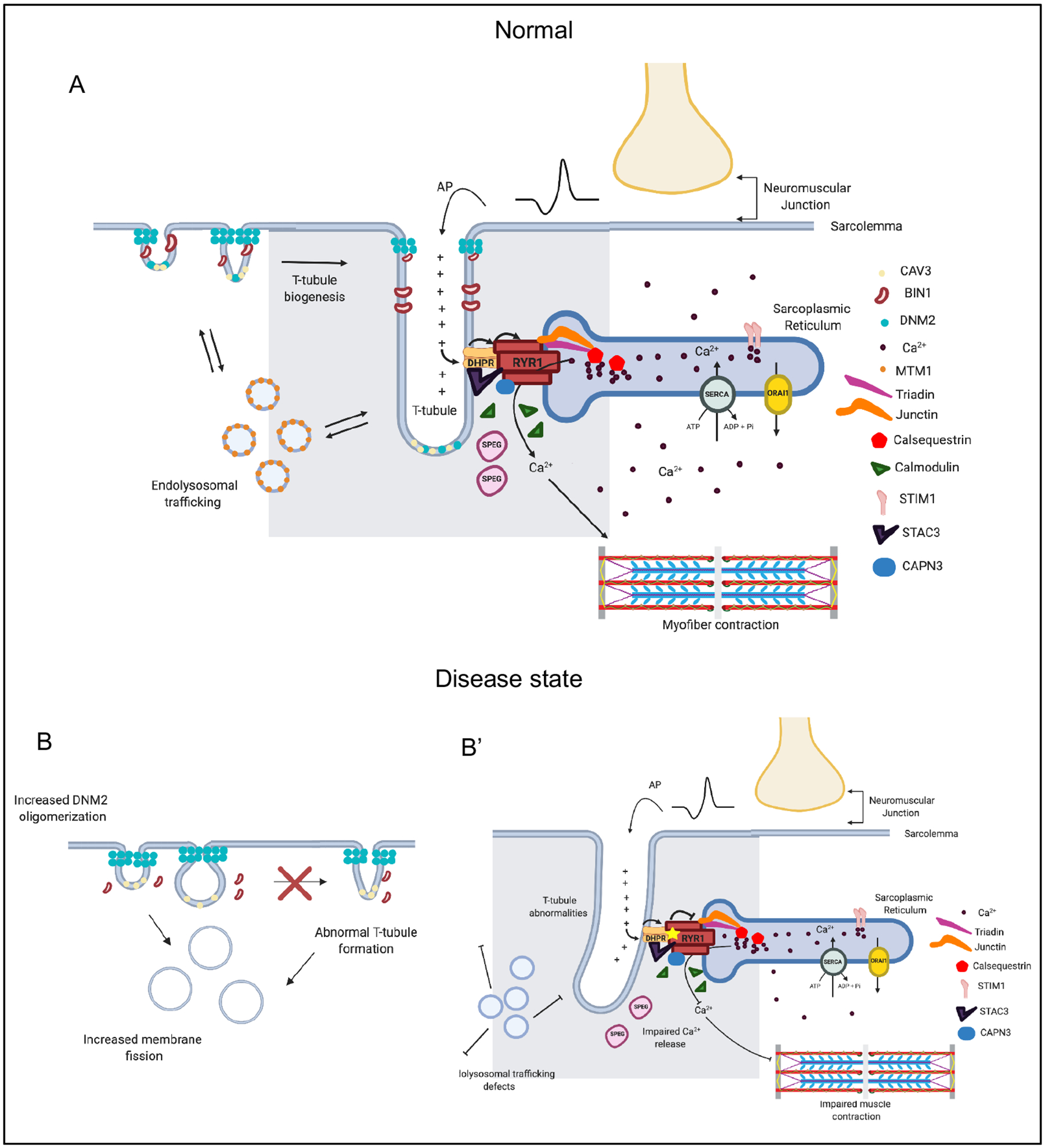
The triad represents the apposition of a Transverse (T)-tubule with two terminal cisternae of the sarcoplasmic reticulum (SR). While many types of proteins and regulatory structures located in or around the triad have been identified, the mechanisms of T-tubule biogenesis and triad formation are still incompletely understood. a | A muscle fiber is excited by the motor neuron at the neuromuscular junction, inducing membrane depolarization, which travels along the sarcolemma and into the t-tubule. The dihydropyridine receptor (DHPR) senses membrane depolarization, and undergoes a conformation change which activates the ryanodine receptor (RYR1), releasing Ca2+ from the SR into the sarcoplasm. Sarcoplasmic Ca2+ then binds to the troponin complex, releasing inhibition and initiating a muscle contraction. Several other proteins are critical for the formation and maintenance of the triad. MTM1 potentially regulates transport of muscle-specific proteins to the triad. BIN1 is involved in membrane remodelling, and in concert with caveole, initiates T tubule formation. DNM2 is speculated to function in parallel (and potentially opposed) to BIN1, via its membrane fission activity, to modulate T-tubule formation and maintenance. Two examples of mutations and their consequences on the triad: b | DNM2 hyperactivity leads to aberrant/premature membrane fission and abnormal t-tubule formation; b’ | RYR1 mutations lead to impaired calcium release and reduced muscle contraction.
