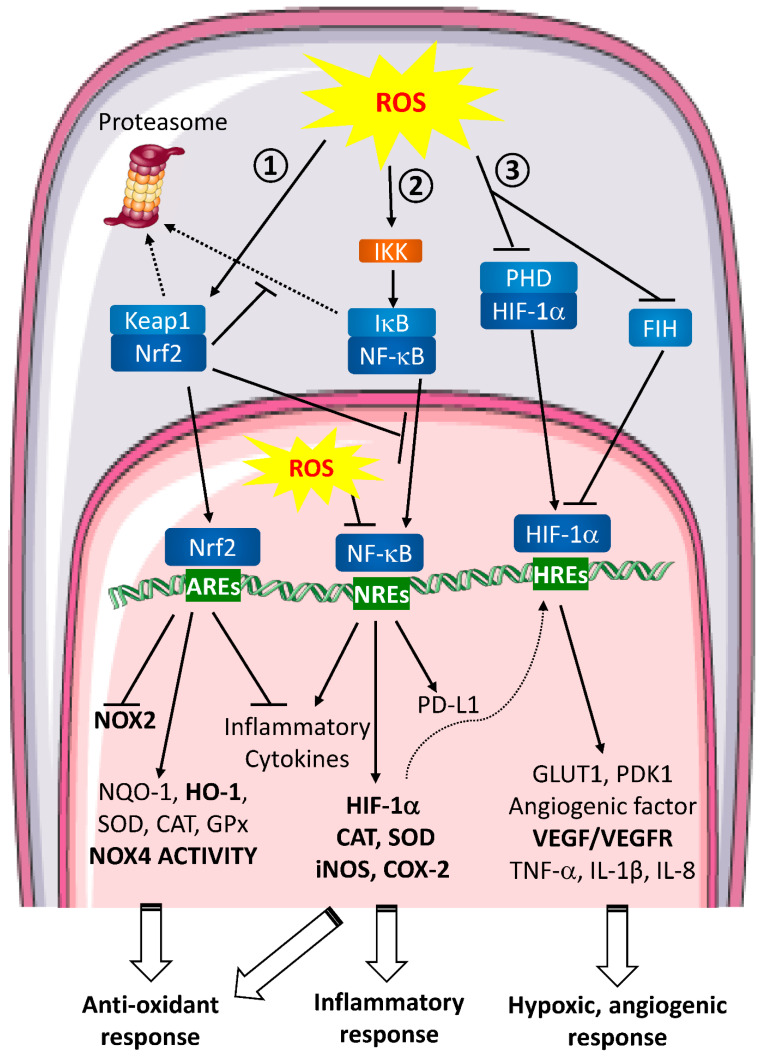
Figure 2.
ROS signaling pathways involved in wound healing and metastasis. Extracellular ROS activate intracellular signaling pathways. ① Nrf2 pathway. In unstressed conditions, Keap1 retains Nrf2 in the cytoplasm. When ROS are produced, Keap1 is oxidized and ubiquitinated thereby leading to its proteasomal degradation. Consequently, Nrf2 is free to translocate to the nucleus and binds to the anti-oxidant response elements (AREs). This binding inhibits NOX2 and pro-inflammatory cytokines transcription and enhances the anti-oxidant defense response expression. ② NF-κB pathway. In normal conditions, NF-κB is associated with IκB and retained in the cytoplasm. In the presence of ROS, IKK is activated and can phosphorylate IκB to induce its dissociation with NF-κB and its proteasomal degradation. Then, free NF-κB translocates to the nucleus, binds to NF-κB Response Elements (NREs) and induces target genes transcription leading to a global inflammatory response. ROS are able to directly act in the nucleus inhibiting NF-κB binding to the NREs. ③ HIF pathway. In homeostatic conditions, HIF-1α is hydroxylated by PHDs and targeted for proteasomal degradation. HIF-1α is also regulated by FIH, which blocks the interaction between HIF-1α transactivation domain and coactivators on HREs. During hypoxia or when ROS are produced, PHDs are inactivated which stabilizes HIF-1α and FIH is inhibited. HIF-1α then translocates into the nucleus where it binds to HIF Response Elements (HREs). Transcription of target genes is induced leading to hypoxic and angiogenic response. Nrf2, NF-κB and HIF pathways are closely interlinked. Nrf2 can inhibit IκB proteasomal degradation and NF-κB nuclear translocation, NF-κB pathway induces anti-oxidant response regulating iNOS and COX-2 transcription and HIF-1α expression is regulated by NF-κB.