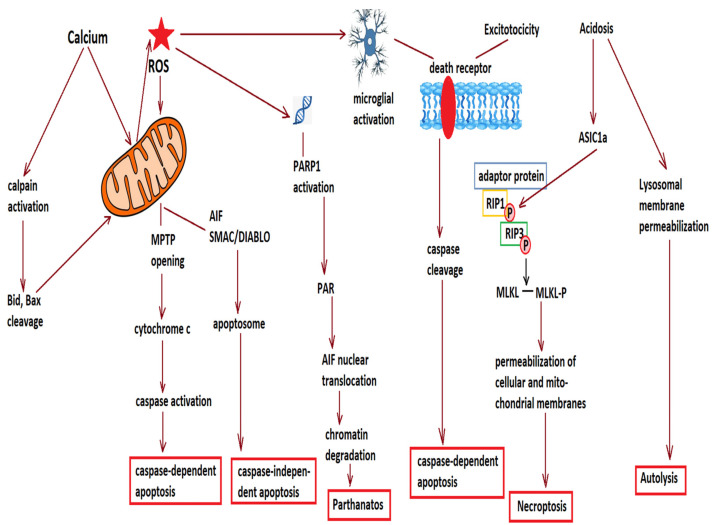
Figure 1.
Various mechanisms contribute to cell loss in the penumbral area. Reactive oxygen species (ROS) and increased cytosolic calcium damage mitochondria, and lead to opening of the mitochondrial permeability transition pore (MPTP) and release of cytochrome c and apoptosis inducing factor (AIF). Increased calcium also activates calpains, which cleave pro-apoptotic factors such as Bid and Bax, promoting their mitochondrial translocation and further permeabilization of the mitochondrial membrane. Cytochrome c activates the caspase cascade and leads to caspase-dependent apoptosis, while AIF and second mitochondrion-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (SMAC/DIABLO) activate caspase-independent apoptosis. ROS also damage DNA and activate poly(ADP-ribose) polymerases (PARP), leading to the production of poly(ADP-ribose) or PAR, which promotes the nuclear translocation of AIF and chromatin degradation, leading to parthanatos. Another consequence of ROS production is microglial activation, which together with excessive stimulation of N-methyl-D-aspartate receptors (excitotoxicity) activate the membrane death receptors, leading to caspase cleavage, as well as to receptor interacting kinase 1 (RIP1) phosphorylation, followed by the phosphorylation of RIP3 and mixed lineage kinase domain-like (MLKL), leading to oligomerization of phosphorylated MLKL at plasma membranes and cell rupture (necroptosis). Acidification, common in the penumbral area of cerebral infarction, promotes the association of acid-sensing ion channel 1a (ASIC1a) with RIP1 and the activation of the latter. In addition, an acidic environment, together with calpain activation, permeabilizes the lysosomal membrane and allows for the release of lysosomal proteases, cathepsins, and hydrolases into the cytosol, leading to autolysis.