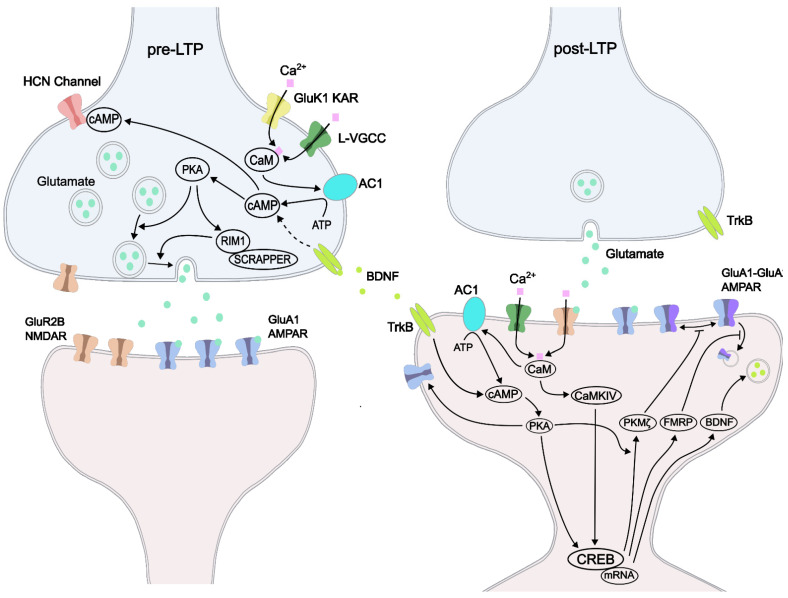
Figure 3.
ACC pre-LTP and post-LTP. Two forms of LTP (pre and post) can be observed in the ACC. Presynaptic enhancement of vesicle formation, pool size, and neurotransmitter release probability lead to more readily available agonists of ionotropic glutamate receptors in the synaptic cleft. This can quickly saturate postsynaptic receptors leading to increased calcium influx through the postsynaptic membrane. GluK1-containing kainate receptors (KARs) and ligand-voltage-gated calcium channels (L-VGCCs) allow for the influx of Ca2+, which activates calmodulin (CaM). Activation of adenylyl cyclase 1 (AC1) by CaM leads to cAMP and protein kinase A (PKA), which enhances vesicle fusion. Regulating synaptic membrane exocytosis protein 1 (RIM1) together with SCRAPPER, a synapse-localized E3 ubiquitin ligase can upregulate synaptic vesicle release. In addition, activity of the HCN channel with cAMP is believed to modulate the spontaneous release of neurotransmitters. Post-LTP requires the activation of postsynaptic NMDARs, which through the influx of Ca2+ can activate the CaMKIV, which activates the cAMP response element-binding protein (CREB). Once activated by cAMP, PKA upregulates the insertion of Ca2+ permeable AMPARs to the membrane surface and phosphorylates CREB leading to increased protein synthesis of PKMζ, fragile x mental retardation protein (FMRP), and brain-derived neurotrophic factor (BDNF). BDNF interacts with presynaptic and postsynaptic TrkB receptors and contributes to LTP via the AC1-PKA pathway. De novo synthesis of proteins contributes to increased survival, synaptic plasticity, and neuronal growth, which can persist over a long period of time. As with the importance of PKA, activation of AC1 is also critical for the maintenance of post-LTP.