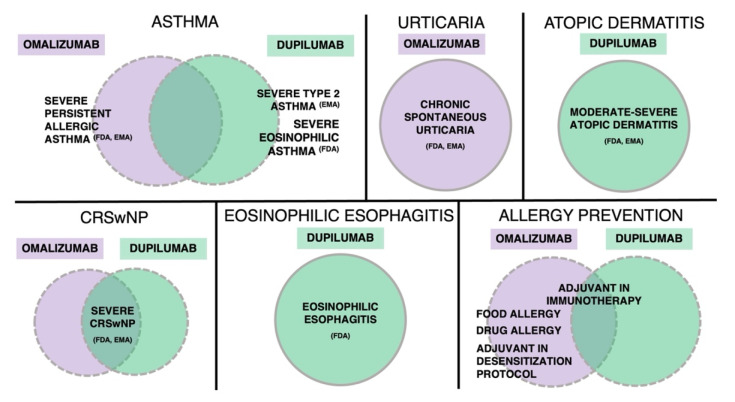
Figure 1.
On-label indications and off-label promising uses of omalizumab and dupilumab in allergic inflammation (as of October 2022). Omalizumab is approved as an add-on treatment in severe persistent allergic asthma (FDA, EMA), similarly to dupilumab in severe type 2 asthma (EMA) and asthma with eosinophilic phenotype (FDA) or oral corticosteroid-dependent asthma, regardless of phenotype (FDA). Regarding allergic skin diseases, omalizumab is approved for the treatment of chronic spontaneous urticaria (FDA, EMA), similarly to dupilumab for the treatment of moderate-severe atopic dermatitis (FDA, EMA). Both biologics have been approved in patients with severe chronic rhinosinusitis with nasal polyps (FDA, EMA). In eosinophilic esophagitis, dupilumab has recently been approved by the FDA. Considering prevention of allergic reactions, both biologics are under investigation as adjuvant in oral immunotherapy. Omalizumab is used off-label as adjuvant in desensitization protocols to prevent breakthrough reactions. FDA denotes U.S. Food and Drug Administration, EMA European Medicines Agency.