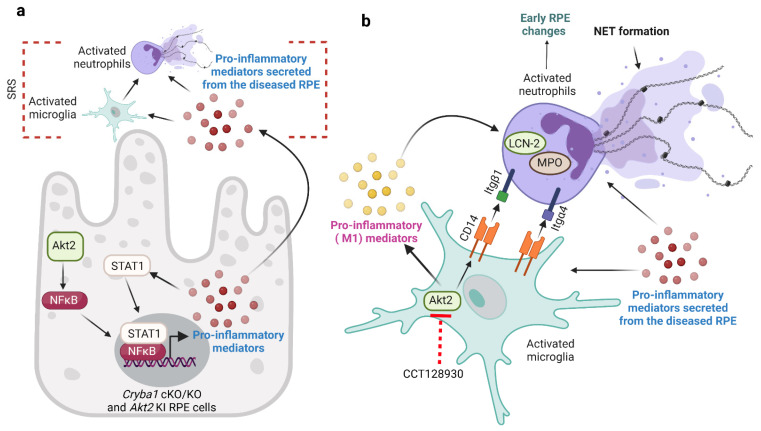
Figure 7.
Microglia–neutrophil interaction in AMD pathogenesis. (a) RPE cells from aged Cryba1 cKO and Akt2 KI mice secrete pro-inflammatory mediators (cytokines and chemokines) due to activation of AKT serine/threonine kinase 2 (Akt2)/nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling. These pro-inflammatory mediators trigger microglia and neutrophils infiltration into the subretinal space (SRS). (b) Cytokines and chemokines secreted by the RPE cells induce pro-inflammatory (M1) transition in microglia through Akt2 activation. These activated microglia release pro-inflammatory mediators and express high levels of cluster of differentiation 14 (CD14) on the cell surface, which in turn activates neutrophils as evident from elevated lipocalin-2 (LCN-2), myeloperoxidase (MPO), and neutrophil extracellular trap (NET) formation, thereby inducing early retinal pigmented epithelium (RPE) changes in nonobese diabetic/severe combined immunodeficiency (NOD-SCID) mice. Further, the M1 microglia can regulate the expression of adhesion proteins such as integrin beta 1 (Itgβ1) and integrin alpha 4 (Itgα4) on neutrophils through their interactions with the high levels of CD14 on the surface of microglia. Interestingly, inhibiting Akt2 in microglia reduced the release of pro-inflammatory cytokines and reduced CD14 levels in these cells, diminishing neutrophil activation in vitro and subsequent changes in morphology in RPE morphology in vivo. Created with BioRender.com (accessed on 30 August 2022).