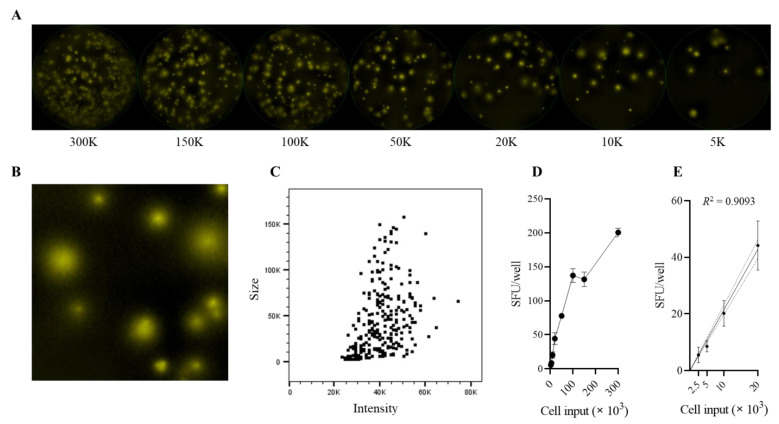
Figure 1.
Accurate measurement of SARS-CoV-2 spike (S1)-reactive ASC frequencies. PBMCs from COVID-19 Donor 1 were pre-stimulated for 5 days in vitro to transition resting B cells, irrespective of their specificity, to antibody-secreting cells (ASCs). PBMCs were then seeded into SARS-CoV-2 S1-coated wells with decreasing cell inputs, starting at 3 × 105 PBMC/well, with increasing numbers of replicate wells. During the overnight culture period, antibodies originating from S1-reactive ASCs were captured on the antigen-coated membrane in close proximity, and the resulting antibody secretory footprints, or spot-forming units (SFUs), were then detected as described in Materials and Methods. (A) Representative well images are shown for the specified cell numbers plated per well (an overview image depicting all replicate wells for COVID-19 Donor 1 is shown in Figure S2). (B) Magnification of a representative well image seeded with 2 × 104 PBMC/well in which individual SFUs are clearly discernable and exhibit variable sizes and fluorescent intensities. (C) SARS-CoV-2 S1-reactive secretory footprints, originating from replicate wells seeded with 2 × 104 PBMCs, were merged into a flow cytometry standard (FCS) file and visualized as a bivariate plot depicting the fluorescence intensity (x-axis) and size (y-axis) of the individual FluoroSpots. (D) Mean ± SD of SARS-CoV-2 S1-reactive SFU counts as a function of cell input. Note the deviation in linearity for SFU counts originating from wells seeded with greater than 2 × 104 PBMC/well inputs. (E) Linearity of SARS-CoV-2 S1-reactive SFU counts originating from wells seeded with less than 2 × 104 PBMC/well. Extrapolation of the regression curve was used to establish the frequency of S1-reactive IgG+ B cells in COVID-19 Donor 1 at 195 SFU/105 PBMC.