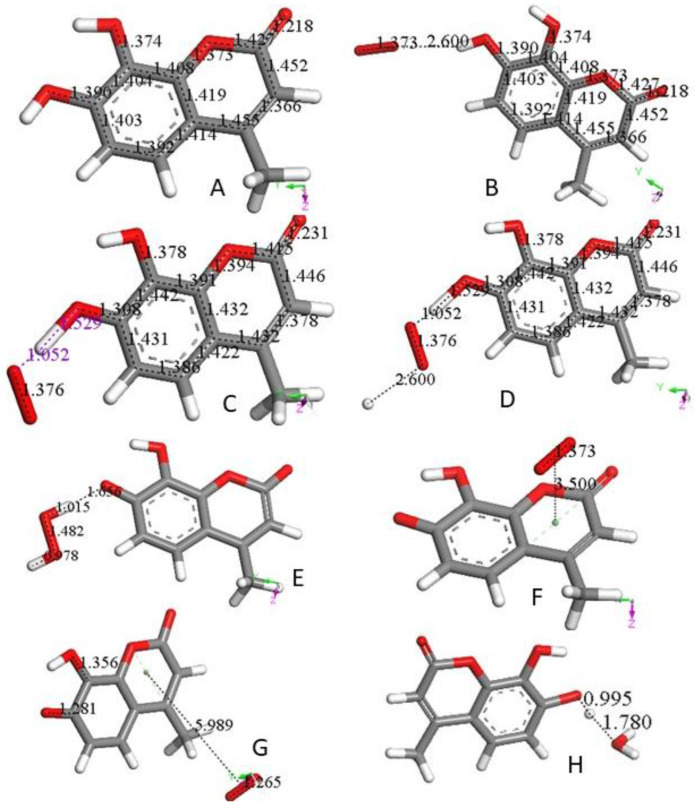
Figure 1.
(A) 4-Methyl-7,8-di-hydroxy-coumarin was geometry optimized. C–C and C–O bonds are displayed for comparison in following figures. (B) A superoxide molecule (O–O bond distance 1.373 Å) was van der Waals posed by H7, 2.60 Å. (C) After geometry optimization of the arrangement shown in (B) O7–H7 breaks (1.529 Å), and H7 is captured by superoxide (1.052 Å). (D) A proton is van der Waals posed near the superoxide, 2.60 Å in the arrangement shown in (C). (E) Geometry optimization of (D) structure results in formation of H2O2, well separated from O7(polyphenol), 1.656 Å. This was followed by H2O2 elimination, and minimization of the remaining neutral coumarin radical. (F) A superoxide radical is π-π posed on top of the pyrone ring (van der Waals separation, 3.50 Å). (G) Geometry optimization of (F) arrangement shows formation of a O2 molecule (O–O bond distance 1.265 Å, much shorter than the 1.373 Å for superoxide), which is about 6 Å from the coumarin species. Bond distance C7–O7, 1.281 Å, is shortened compared with that in Figure 1A, 1.396. This is due to the double bond character of the former compared with the single bond of the latter. (H) After elimination of O2 from (F) a hydronium ion is van der Waals posed close to O7, 2.60 Å, making the whole charge of the system neutral, and upon geometry optimization a proton is captured by the coumarin (C7–H = 0.995 Å), while a molecule of H2O, well separated from the polyphenol (1.780 Å), forms. That is, the coumarin molecule is reformed, becoming ready for an additional SOD-like cycle. If instead of a hydronium, a proton is reacted, the same result (coumarin formation) is obtained.