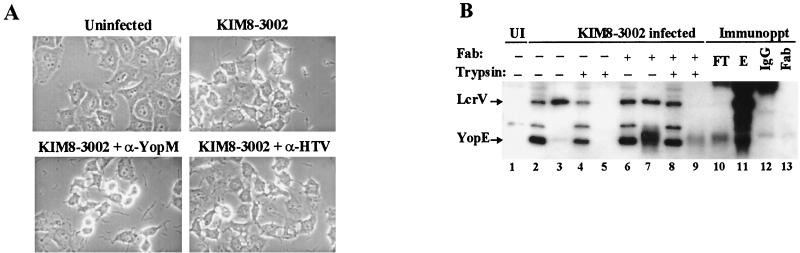
FIG. 8.
LcrV-specific antibody does not neutralize Yop-targeting activity by nonpreinduced Y. pestis. (A) α-HTV or α-YopM antibody was added at 175 μg/ml to warm RPMI containing Y. pestis KIM8-3002, and the mixture was added without centrifugation to monolayers of HeLa cells at an MOI of 10. After incubation for 3 h at 37°C with 5% CO2, the cultures were visualized by phase-contrast microscopy and photographed through a green filter. (B) HeLa cell monolayers were untreated (UI) (lane 1) or infected with Y. pestis KIM8-3002 at an MOI of 10 in the presence (lanes 6 to 9) or absence (lanes 2 to 5) of LcrV-specific Fab antibody fragments (α-HTV Fab). For this experiment, Fab antibody fragments were present at 26.5 μg/ml, corresponding to a 1,000-fold molar excess over estimated LcrV levels. After 4 h, replicate wells were treated with trypsin at 100 μg/ml prior to harvesting (lanes 4, 5, 8, and 9) or were harvested directly. Samples were fractionated into cell-free supernatants (lanes 3, 5, 7, and 9) and HeLa cell soluble fractions (lanes 1, 2, 4, 6, and 8). To verify that released LcrV could be quantitatively bound by the Fab fragments, LcrV bound by α-HTV Fab was immunoprecipitated (Immunoppt) from a nontrypsinized supernatant fraction by using anti-IgG and protein A-agarose beads. The combined void and protein A column washes (FT), elution (E) fractions, and fractionated culture samples were analyzed by immunoblotting with α-HTV and α-YopE. Preparations of whole antibody (IgG) (lane 12) and Fab fragments (lane 13) were also resolved as references. Proteins were visualized by probing with horseradish peroxidase-coupled secondary antibody, developed with ECL reagent, and exposed to film.