Abstract
Background
The impact of the COVID-19 pandemic on the incidence and management of inflammatory arthritis is not understood. Routinely captured data in secure platforms, such as OpenSAFELY, offer unique opportunities to understand how care for patients with inflammatory arthritis was impacted upon by the pandemic. Our objective was to use OpenSAFELY to assess the effects of the pandemic on diagnostic incidence and care delivery for inflammatory arthritis in England and to replicate key metrics from the National Early Inflammatory Arthritis Audit.
Methods
In this population-level cohort study, we used primary care and hospital data for 17·7 million adults registered with general practices using TPP health record software, to explore the following outcomes between April 1, 2019, and March 31, 2022: (1) incidence of inflammatory arthritis diagnoses (rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis, and undifferentiated inflammatory arthritis) recorded in primary care; (2) time to first rheumatology assessment; (3) time to first prescription of a disease-modifying antirheumatic drug (DMARD) in primary care; and (4) choice of first DMARD.
Findings
Among 17 683 500 adults, there were 31 280 incident inflammatory arthritis diagnoses recorded between April 1, 2019, and March 31, 2022. The mean age of diagnosed patients was 55·4 years (SD 16·6), 18 615 (59·5%) were female, 12 665 (40·5%) were male, and 22 925 (88·3%) of 25 960 with available ethnicity data were White. New inflammatory arthritis diagnoses decreased by 20·3% in the year commencing April, 2020, relative to the preceding year (5·1 vs 6·4 diagnoses per 10 000 adults). The median time to first rheumatology assessment was shorter during the pandemic (18 days; IQR 8–35) than before (21 days; 9–41). The proportion of patients prescribed DMARDs in primary care was similar before and during the pandemic; however, during the pandemic, fewer people were prescribed methotrexate or leflunomide, and more were prescribed sulfasalazine or hydroxychloroquine.
Interpretation
Inflammatory arthritis diagnoses decreased markedly during the early phase of the pandemic. The impact on rheumatology assessment times and DMARD prescribing in primary care was less marked than might have been anticipated. This study demonstrates the feasibility of using routinely captured, near real-time data in the secure OpenSAFELY platform to benchmark care quality on a national scale, without the need for manual data collection.
Funding
None.
Introduction
Autoimmune inflammatory arthritis encompasses an overlapping group of conditions that include rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis, and undifferentiated inflammatory arthritis. Early diagnosis of inflammatory arthritis, and prompt treatment with disease-modifying antirheumatic drugs (DMARDs), improves outcomes for patients and increases the likelihood of remission.1, 2, 3
The COVID-19 pandemic placed enormous strain on health-care services and their ability to deliver optimal care for patients with chronic conditions.4 In the UK, primary care referrals decreased by over 50% during the pandemic; hospital outpatient services transitioned to virtual consultations; and individuals delayed seeking care due to fear of infection or burdening services.5, 6, 7, 8 In the USA, reductions in rheumatology visits were observed, with increased use of telemedicine.9, 10 In Sweden, a 7% decrease in the incidence of inflammatory joint diseases was seen in 2020, relative to 2015–19, continuing an overall downward trend in incidence; additionally, modest reductions in rheumatology visits (–16%) and conventional synthetic DMARD dispensations (–8·5%) were reported during the pandemic, while biologic and targeted synthetic DMARD dispensations increased by 6·5%.11
Research in context.
Evidence before this study
We performed a literature search for population-level, observational cohort studies that compared the incidence or management of inflammatory arthritis diagnoses (rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis, or undifferentiated inflammatory arthritis) during and before the COVID-19 pandemic. We searched PubMed for articles published from the date of database inception to Sept 8, 2022, with no language restrictions, using the terms “incidence”, “assessment”, “management”, “treatment”, “arthritis”, “spondyloarthritis”, and “COVID”. A study in Sweden reported a 7% decrease in the incidence of inflammatory joint diseases during the pandemic, in addition to a 16% reduction in rheumatology visits, 8·5% decrease in conventional synthetic disease-modifying antirheumatic drug (DMARD) dispensations, and 6·5% increase in biological and targeted synthetic DMARD dispensations. Two US-based studies used electronic health record data from participating rheumatology practices to demonstrate reduced rheumatology visits during the pandemic and increased telemedicine use.
Added value of this study
Our study used the OpenSAFELY data platform of 17·7 million adults (representing 40% of the English population) to compare incident diagnoses and care delivery for patients with inflammatory arthritis between April 1, 2019, and March 31, 2022. We found that the monthly incidence of inflammatory arthritis diagnoses decreased by 40% early in the pandemic. Further decreases coincided with rising COVID-19 numbers, before returning to prepandemic levels by the end of the study period. Overall, in the year commencing April, 2020, there was a 20% decrease in inflammatory arthritis diagnoses relative to the preceding year. For people who sought medical care for new inflammatory arthritis, we showed that the time to first rheumatology assessment was shorter during the pandemic than before. The proportion of patients prescribed conventional synthetic DMARDs in primary care was similar, although fewer patients were prescribed methotrexate or leflunomide during the pandemic, and more were prescribed sulfasalazine or hydroxychloroquine.
Implications of all the available evidence
There were marked decreases in new inflammatory arthritis diagnoses during the pandemic, which coincided with rising COVID-19 case numbers in England. No rebound increase in incidence above prepandemic levels was observed in later months, indicating that there is likely to be a substantial burden of undiagnosed inflammatory arthritis as a consequence of the pandemic. For people who sought medical care, the impact of the pandemic on rheumatology assessment times and DMARD prescribing in primary care was less marked than might have been expected. Our study demonstrates the feasibility of using routinely captured data in the secure OpenSAFELY platform to benchmark care quality on a national scale and without the need for manual data collection.
In England and Wales, the Healthcare Quality Improvement Partnership (HQIP) commissions national audit programmes, with the aim of monitoring health-care services and improving outcomes.12 The National Early Inflammatory Arthritis Audit (NEIAA) is the largest audit of its kind globally, reporting annually on care delivered across all National Health Service (NHS) rheumatology services in England and Wales.13 In NEIAA, hospitals are benchmarked against National Institute for Health and Care Excellence (NICE) guidance and other indicators of care quality.13, 14 Metrics include time from primary care referral to initial rheumatology assessment, and time to initiation of a DMARD.15 Data for NEIAA are entered manually by participating hospitals; however, data capture is often incomplete, especially in underperforming units where poor engagement in a national audit programme correlates with the quality of care provided.16 Mandatory data collection in NEIAA was paused during the pandemic, preventing comparisons of care.
OpenSAFELY is a secure data analytics platform for electronic health records (EHRs), built with the approval of NHS England to deliver urgent research and health service evaluation on the direct and indirect impacts of the pandemic.17 It was created as a collaboration between the University of Oxford DataLab, the London School of Hygiene & Tropical Medicine, TPP (a software provider for general practitioners), and NHS England. OpenSAFELY provides a secure software interface, enabling analyses of pseudonymised health records in near real-time within the EHR vendor's highly secure data centre, avoiding the need for data transfer off-site. Pseudonymised data, such as coded diagnoses and medications, are included in OpenSAFELY, but free-text data are not included. Analyses can run across individuals’ full, raw, pseudonymised EHRs at 99% of English general practices, including patient-level linkage to secondary care data.
Our objective was to use OpenSAFELY to replicate key metrics from NEIAA and to assess the impact of COVID-19 on care delivery for people with inflammatory arthritis in England.
Methods
Study design and participants
We did a cohort study using EHR data. We compared the incidence of inflammatory arthritis diagnoses in primary care, and timing of assessment and treatment for people with incident inflammatory arthritis, before and after the onset of the COVID-19 pandemic in England.
Due to data availability, we piloted our approach in OpenSAFELY-TPP, which contains data for 24 million people registered with general practices using TPP SystmOne software (approximately 40% of the English population). OpenSAFELY-TPP is representative of the English population in terms of age, sex, index of multiple deprivation (IMD), ethnicity, and causes of death.18 Primary care records were linked to NHS Secondary Uses Service data through OpenSAFELY.
The reference population consisted of all adults, aged 18–110 years, registered with practices using TPP software in England for at least 12 months as of April 1, 2019. From this population, we defined the inflammatory arthritis cohort as people with index diagnostic codes for rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis, or undifferentiated inflammatory arthritis between April 1, 2019, and March 31, 2022 (see appendix p 11 for codelists).
We defined the index diagnosis date as the first appearance of an inflammatory arthritis code in the primary care record. At least 12 months of continuous registration before the diagnosis date was required to ensure only index diagnoses were captured. People with new inflammatory arthritis diagnostic codes who had received prescriptions for conventional synthetic DMARDs (eg, methotrexate) or biological DMARDs (eg, adalimumab) 60 days or more before their first rheumatology appointment were deemed not to be new diagnoses and were excluded from analyses (n=4880). For individuals in whom the inflammatory arthritis subdiagnosis subsequently changed (eg, from undifferentiated inflammatory arthritis to rheumatoid arthritis), the most recent subdiagnosis was selected as the final diagnosis.
Baseline sociodemographic characteristics and comorbidities were described without inferential statistics for the inflammatory arthritis cohort (at the time of diagnosis) and the reference population (at April 1, 2019), as follows: age, sex, ethnicity (White, Asian or Asian British, Black, mixed or other), deprivation (IMD quintiles: from 1, most deprived, to 5, least deprived), smoking status (current, former, never), obesity (categorised according to the most recent BMI), hypertension, diabetes, stroke, chronic cardiac disease, chronic respiratory disease, chronic liver disease, cancer, and chronic kidney disease (defined as an estimated glomerular filtration rate <60 mL/min/1·73 m2 or a diagnostic code for end-stage renal failure). Further details of comorbidity definitions and codelists are included in the appendix (p 11).
Approval to undertake this study under the remit of service evaluation was obtained from King's College Hospital NHS Foundation Trust. No further ethical approval was required as per UK Health Research Authority guidance. An information governance statement is included in the appendix (p 12).
Outcomes
The incidence of inflammatory arthritis diagnoses was calculated by dividing the number of new inflammatory arthritis diagnoses in primary care during each study year (April 1, 2019, to March 31, 2020; April 1, 2020, to March 31, 2021; April 1, 2021, to March 31, 2022) by the number of people in the reference population.
For people within the inflammatory arthritis cohort who had their first rheumatology outpatient attendance captured and who had at least 12 months of available follow-up, we documented the following outcomes: median time (in days) from primary care referral to initial rheumatology assessment; median time (in days) from initial rheumatology assessment to first prescription of a conventional synthetic DMARD in primary care; and choice of first conventional synthetic DMARD. Among people who had an initial rheumatology assessment, we additionally recorded the method (in-person or virtual) of consultation.
In England, guidelines recommend that individuals who present to their general practitioner with symptoms suggestive of a new inflammatory arthritis diagnosis should be referred to a rheumatology specialist, and rheumatology assessment should occur within 3 weeks of referral.14, 19, 20 Specialist assessment usually occurs in a rheumatology outpatient service affiliated with a hospital. Where indicated, DMARDs are initiated by the rheumatology specialist in secondary care (dispensed by hospital pharmacies). Once established on a stable dose, prescribing of conventional synthetic DMARDs typically transitions to primary care prescribing (dispensed by community pharmacies) as a shared-care responsibility between primary and secondary care.21
We defined the initial rheumatology assessment as the date of first attendance at a rheumatology outpatient clinic (defined by the “410” treatment function code22). If the first appointment was not captured within 12 months before the index diagnostic code appeared in the primary care record, it was looked for within 60 days after the index diagnosis. We defined the primary care referral date as the last primary care assessment (virtual or in-person) before the first rheumatology appointment.
We defined the prescription of a conventional synthetic DMARD in primary care as at least one prescription issued for methotrexate (oral or subcutaneous), hydroxychloroquine, sulfasalazine, or leflunomide. Only primary care prescriptions were captured; prescriptions dispensed by hospital pharmacies were not captured. An upper limit of 12 months after the first rheumatology appointment was used for prescriptions, to minimise bias from unequal follow-up time between individuals entering the cohort at different times.
Statistical analysis
Assessment and treatment outcomes were presented by year and by region (categorised into the nine Nomenclature of Territorial Units for Statistics level 1 regions within England23). Interrupted time-series analyses were used to estimate the impact of the pandemic on the proportion of patients with incident inflammatory arthritis (averaged by month) who: (1) were assessed by rheumatology within 3 weeks of primary care referral; and (2) were prescribed a conventional synthetic DMARD in primary care within 6 months of initial rheumatology assessment. Trends in these outcomes were compared before and after the first COVID-19 lockdown in England (March, 2020) using single-group interrupted time-series analyses.24 Newey-West standard errors with five lags were used to account for autocorrelation between observation periods.
Python 3.8 was used for data management. Stata version 16 was used for statistical analyses. As the primary objective of our analyses was descriptive, no correction for multiple hypothesis testing was performed. For statistical disclosure control, we rounded frequency counts to the nearest 5 and redacted non-zero counts below 6. For conventional synthetic DMARD prescribing in individuals with axial spondyloarthritis, only overall counts were presented (ie, not by region or study year) due to small numbers. Code for data management and analysis is shared openly for review and reuse under MIT open license. Detailed pseudonymised patient data are potentially reidentifiable and therefore are not shared.
Patient and public involvement
OpenSAFELY has a publicly available website through which we invite patients or members of the public to contact us about this study or the broader OpenSAFELY project.
Role of the funding source
There was no direct funding source for this study.
Results
Between April 1, 2019, and March 31, 2022, there were 31 280 incident inflammatory arthritis diagnoses recorded in primary care from a reference population of 17 683 500 people aged 18 years or older. Of new inflammatory arthritis diagnoses, 19 085 (61·0%) were rheumatoid arthritis, 6825 (21·8%) were psoriatic arthritis, 3970 (12·7%) were axial spondyloarthritis, and 1400 (4·5%) were undifferentiated inflammatory arthritis. A flow diagram of study populations is shown in the appendix (p 8).
Baseline characteristics of people with incident inflammatory arthritis, compared with the reference population, are shown in the table . The mean age at diagnosis of people with inflammatory arthritis was 55·4 years (SD 16·6): 60·4 years (15·4) for rheumatoid arthritis, 48·6 years (14·4) for psoriatic arthritis, 43·2 years (15·6) for axial spondyloarthritis, and 55·2 years (17·3) for undifferentiated inflammatory arthritis. 18 615 (59·5%) of 31 280 patients with inflammatory arthritis were female (12 385 [64·9%] of 19 085 patients with rheumatoid arthritis; 3675 [53·8%] of 6825 patients with psoriatic arthritis; 1750 [44·1%] of 3970 patients with axial spondyloarthritis; and 805 [57·5%] of 1400 patients with undifferentiated inflammatory arthritis). 22 925 (88·3%) of 25 960 patients with inflammatory arthritis (and available data) were White (13 825 [87·2%] of 15 850 patients with rheumatoid arthritis; 5175 [90·9%] of 5690 patients with psoriatic arthritis; 2900 [88·5%] of 3275 patients with axial spondyloarthritis; and 1025 [89·5%] of 1145 patients with undifferentiated inflammatory arthritis).
Table.
Baseline demographics and comorbidities for people with incident inflammatory arthritis diagnoses, overall and separated into subdiagnoses, compared with the reference population
| General population (n=17 683 500) | All inflammatory arthritis (n=31 280) | Rheumatoid arthritis (n=19 085) | Psoriatic arthritis (n=6825) | Axial spondyloarthritis (n=3970) | Undifferentiated inflammatory arthritis (n=1400) | ||
|---|---|---|---|---|---|---|---|
| Age group, years | |||||||
| 18–39 | 6 049 070 (34·2%) | 6225 (19·9%) | 2080 (10·9%) | 1995 (29·2%) | 1865 (47·0%) | 285 (20·4%) | |
| 40–49 | 2 930 905 (16·6%) | 5030 (16·1%) | 2375 (12·4%) | 1555 (22·8%) | 860 (21·7%) | 240 (17·1%) | |
| 50–59 | 3 124 025 (17·7%) | 6935 (22·2%) | 4255 (22·3%) | 1740 (25·5%) | 650 (16·4%) | 290 (20·7%) | |
| 60–69 | 2 455 845 (13·9%) | 5820 (18·6%) | 4310 (22·6%) | 935 (13·7%) | 310 (7·8%) | 260 (18·6%) | |
| 70–79 | 1 971 630 (11·1%) | 5130 (16·4%) | 4240 (22·2%) | 505 (7·4%) | 175 (4·4%) | 205 (14·6%) | |
| ≥80 | 1 152 025 (6·5%) | 2140 (6·8%) | 1825 (9·6%) | 95 (1·4%) | 105 (2·6%) | 120 (8·6%) | |
| Sex | |||||||
| Female | 8 866 535 (50·1%) | 18 615 (59·5%) | 12 385 (64·9%) | 3675 (53·8%) | 1750 (44·1%) | 805 (57·5%) | |
| Male | 8 816 965 (49·9%) | 12 665 (40·5%) | 6700 (35·1%) | 3150 (46·2%) | 2220 (55·9%) | 595 (42·5%) | |
| Ethnicity | |||||||
| White | 12 025 695 (86·6%) | 22 925 (88·3%) | 13 825 (87·2%) | 5175 (90·9%) | 2900 (88·5%) | 1025 (89·5%) | |
| Asian or Asian British | 1 029 955 (7·4%) | 1965 (7·6%) | 1320 (8·3%) | 360 (6·3%) | 220 (6·7%) | 65 (5·7%) | |
| Black | 343 885 (2·5%) | 465 (1·8%) | 350 (2·2%) | 35 (0·6%) | 55 (1·7%) | 25 (2·2%) | |
| Mixed or other | 493 170 (3·5%) | 605 (2·3%) | 355 (2·2%) | 120 (2·1%) | 100 (3·1%) | 30 (2·6%) | |
| Missing | 3 790 795 | 5315 | 3235 | 1130 | 695 | 255 | |
| Index of multiple deprivation | |||||||
| 1 (most deprived) | 3 285 410 (18·9%) | 5660 (18·5%) | 3570 (19·1%) | 1235 (18·5%) | 670 (17·3%) | 185 (13·5%) | |
| 2 | 3 557 860 (20·4%) | 5960 (19·5%) | 3745 (20·0%) | 1250 (18·8%) | 735 (18·9%) | 230 (16·8%) | |
| 3 | 3 762 515 (21·6%) | 6945 (22·7%) | 4270 (22·8%) | 1490 (22·4%) | 870 (22·4%) | 315 (23·0%) | |
| 4 | 3 448 770 (19·8%) | 6385 (20·8%) | 3845 (20·5%) | 1385 (20·8%) | 815 (21·0%) | 340 (24·8%) | |
| 5 (least deprived) | 3 360 490 (19·3%) | 5675 (18·5%) | 3285 (17·6%) | 1300 (19·5%) | 790 (20·4%) | 300 (21·9%) | |
| Missing | 268 455 | 650 | 370 | 165 | 90 | 25 | |
| BMI, kg/m2 | |||||||
| Underweight (<18·5) | 319 635 (2·3%) | 430 (1·6%) | 295 (1·7%) | 60 (1·0%) | 65 (2·0%) | 10 (0·8%) | |
| Healthy weight (18·5–24·9) | 4 850 520 (35·1%) | 7850 (28·6%) | 4880 (28·5%) | 1400 (23·9%) | 1180 (36·8%) | 390 (31·5%) | |
| Overweight (25–29·9) | 4 779 205 (34·6%) | 9530 (34·7%) | 6140 (35·8%) | 1890 (32·2%) | 1055 (32·9%) | 445 (35·9%) | |
| Obese I (30–34·9) | 2 416 505 (17·5%) | 5700 (20·8%) | 3565 (20·8%) | 1330 (22·7%) | 535 (16·7%) | 265 (21·4%) | |
| Obese II (35–39·9) | 926 190 (6·7%) | 2430 (8·8%) | 1405 (8·2%) | 700 (11·9%) | 240 (7·5%) | 85 (6·9%) | |
| Obese III (≥40) | 529 075 (3·8%) | 1520 (5·5%) | 850 (5·0%) | 490 (8·3%) | 135 (4·2%) | 45 (3·6%) | |
| Missing | 3 862 370 | 3815 | 1950 | 950 | 760 | 160 | |
| Smoking status | |||||||
| Never | 8 132 435 (47·9%) | 11 725 (37·9%) | 6905 (36·5%) | 2590 (38·4%) | 1640 (42·5%) | 590 (42·9%) | |
| Former | 5 831 370 (34·3%) | 13 330 (43·1%) | 8530 (45·0%) | 2920 (43·3%) | 1305 (33·9%) | 575 (41·8%) | |
| Current | 3 024 645 (17·8%) | 5 860 (19·0%) | 3500 (18·5%) | 1235 (18·3%) | 910 (23·6%) | 210 (15·3%) | |
| Missing | 695 050 | 360 | 150 | 75 | 110 | 20 | |
| Comorbidities | |||||||
| Hypertension | 3 771 745 (21·3%) | 9005 (28·8%) | 6580 (34·5%) | 1430 (21·0%) | 570 (14·4%) | 420 (30·0%) | |
| Diabetes | |||||||
| No diabetes | 15 973 490 (90·3%) | 26 460 (84·6%) | 15 605 (81·8%) | 5995 (87·8%) | 3660 (92·2%) | 1195 (85·4%) | |
| Diabetes with HbA1c <58 mmol/mol | 1 084 530 (6·1%) | 3270 (10·5%) | 2420 (12·7%) | 525 (7·7%) | 195 (4·9%) | 130 (9·3%) | |
| Diabetes with HbA1c >58 mmol/mol | 498 030 (2·8%) | 1300 (4·2%) | 900 (4·7%) | 255 (3·7%) | 85 (2·1%) | 65 (4·6%) | |
| Diabetes with no HbA1c measure | 127 450 (0·7%) | 250 (0·8%) | 160 (0·8%) | 50 (0·7%) | 30 (0·8%) | 10 (0·7%) | |
| Chronic cardiac disease | 1 192 945 (6·7%) | 2975 (9·5%) | 2285 (12·0%) | 350 (5·1%) | 195 (4·9%) | 145 (10·4%) | |
| Stroke | 370 030 (2·1%) | 815 (2·6%) | 630 (3·3%) | 85 (1·2%) | 60 (1·5%) | 40 (2·9%) | |
| Cancer | 953 380 (5·4%) | 2045 (6·5%) | 1485 (7·8%) | 280 (4·1%) | 145 (3·7%) | 135 (9·6%) | |
| Chronic respiratory disease | 715 820 (4·0%) | 2580 (8·2%) | 2080 (10·9%) | 285 (4·2%) | 130 (3·3%) | 80 (5·7%) | |
| Chronic liver disease | 98 535 (0·6%) | 280 (0·9%) | 160 (0·8%) | 85 (1·2%) | 25 (0·6%) | 10 (0·7%) | |
| Chronic kidney disease | 1 123 905 (6·4%) | 2270 (7·3%) | 1810 (9·5%) | 245 (3·6%) | 110 (2·8%) | 105 (7·5%) | |
Counts have been rounded to the nearest 5, to reduce the risk of disclosure; as such, column totals might differ from the sum of the individual variables. HbA1c=glycated haemoglobin.
People with inflammatory arthritis were more likely to be overweight or obese than the general population (69·8% vs 62·6%); more likely to have a smoking history (62·1% vs 52·1%); and more likely to have hypertension (28·8% vs 21·3%), diabetes (15·5% vs 9·6%), chronic cardiac disease (9·5% vs 6·7%), or chronic respiratory disease (8·2% vs 4·0%).
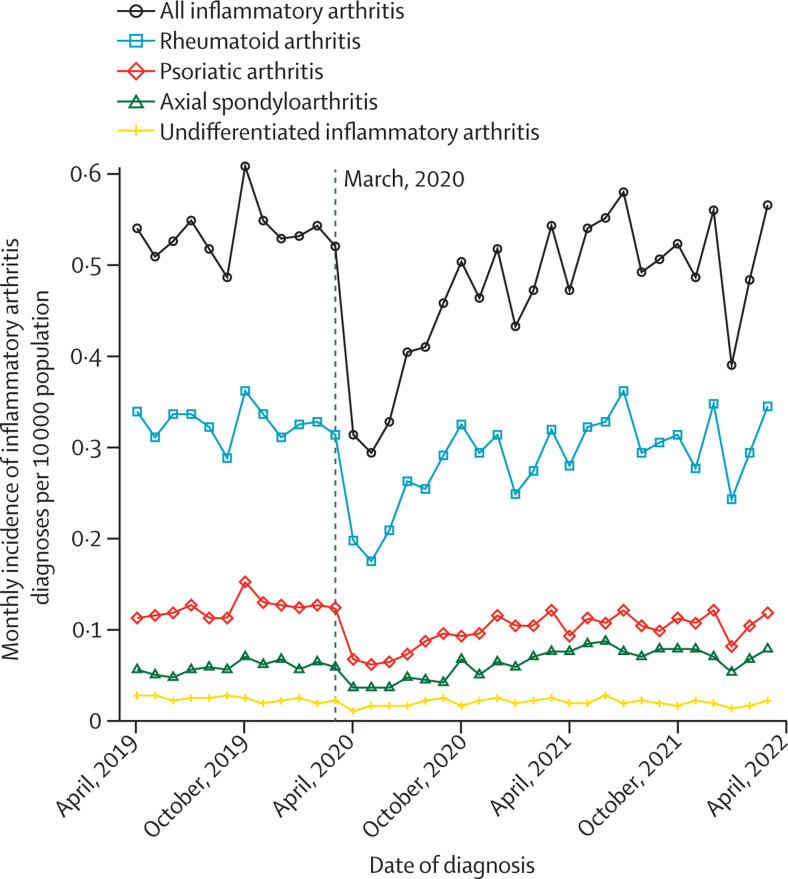
The monthly incidence of recorded inflammatory arthritis diagnoses is shown in figure 1 and the appendix (p 2). Between March and April, 2020 (the start of the first COVID-19 lockdown in England), monthly inflammatory arthritis diagnoses decreased by 39·7%, from 0·52 to 0·31 per 10 000 adults (from 920 diagnoses in March, 2020, to 555 in April, 2020). This was followed by an increase in inflammatory arthritis diagnoses after June, 2020, approaching prepandemic levels by October, 2020. On a background of monthly variations in numbers of diagnoses, the two largest subsequent decreases in inflammatory arthritis diagnoses occurred between December, 2020, and January, 2021 (16·4%; from 915 to 765 diagnoses) and between December, 2021, and January, 2022 (30·3%; from 990 to 690 diagnoses)—coinciding with rising COVID-19 cases in England—before returning to prepandemic levels by the end of the study period. Similar patterns were observed for female and male patients (appendix p 9).
Figure 1.
Incidence of inflammatory arthritis diagnoses recorded in primary care during each month of the study period
The vertical dashed line corresponds to the onset of the first COVID-19 lockdown in England (March, 2020).
The incidence of combined inflammatory arthritis diagnoses by study year was 6·4 per 10 000 adults between April 1, 2019, and March 31, 2020; 5·1 per 10 000 between April 1, 2020, and March 31, 2021; and 6·1 per 10 000 between April 1, 2021, and March 31, 2022. For rheumatoid arthritis, the incidence decreased from 3·9 per 10 000 adults between April 1, 2019, and March 31, 2020, to 3·2 per 10 000 between April 1, 2020, and March 31, 2021, increasing to 3·7 per 10 000 between April 1, 2021, and March 31, 2022. Similar patterns were observed for psoriatic arthritis, axial spondyloarthritis, and undifferentiated inflammatory arthritis, and when separated by sex (appendix pp 3–4).
Of 31 280 incident inflammatory arthritis diagnoses, 20 385 (65·2%) had data captured on their first rheumatology appointment, of whom 19 720 (96·7%) had a primary care appointment captured in the preceding year. The median time from the initial rheumatology appointment to an inflammatory arthritis code appearing in the primary care record was 14 days (IQR 0–84). Of 19 720 patients with data on their first rheumatology appointment and preceding primary care appointment, 13 405 (68·0%) had at least 12 months of available follow-up to enable analyses of assessment times and conventional synthetic DMARD prescribing.
The median time from primary care referral to initial rheumatology assessment was 20 days (IQR 9–38). The median assessment time was shorter for rheumatoid arthritis (17 days; IQR 8–33) than psoriatic arthritis (24 days; IQR 12–49) or axial spondyloarthritis (28 days; IQR 12–69), and similar to undifferentiated inflammatory arthritis (19 days; IQR 8–38).
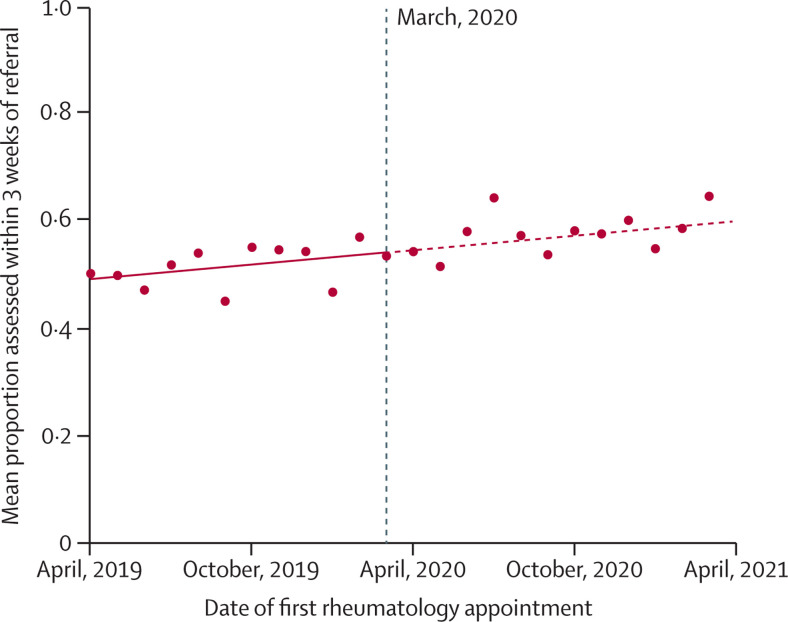
The median time to rheumatology assessment was shorter for patients first assessed after the onset of the pandemic (18 days; IQR 8–35; n=6025) than for patients first assessed before the pandemic (21 days; IQR 9–41; n=7380). Using interrupted time-series analyses, we compared monthly trends in the proportion of patients assessed by rheumatology within 3 weeks of referral (figure 2 ). Improvements in assessment times began before the pandemic and continued during the pandemic, with no significant differences in overall trends: trend pre-March 2020, 0·054% improvement per year (95% CI 0·023 to 0·086); trend post-March 2020, 0·063% improvement per year (95% CI 0·026 to 0·099); difference in trends, 0·009% per year (95% CI –0·043 to 0·060; p=0·73).
Figure 2.
Interrupted time series analysis demonstrating trends in the proportion of patients with incident inflammatory arthritis who were assessed by rheumatology within 3 weeks of primary care referral
Single time point dots represent monthly averages. The vertical dashed line corresponds to the onset of the first COVID-19 lockdown in England (March, 2020).
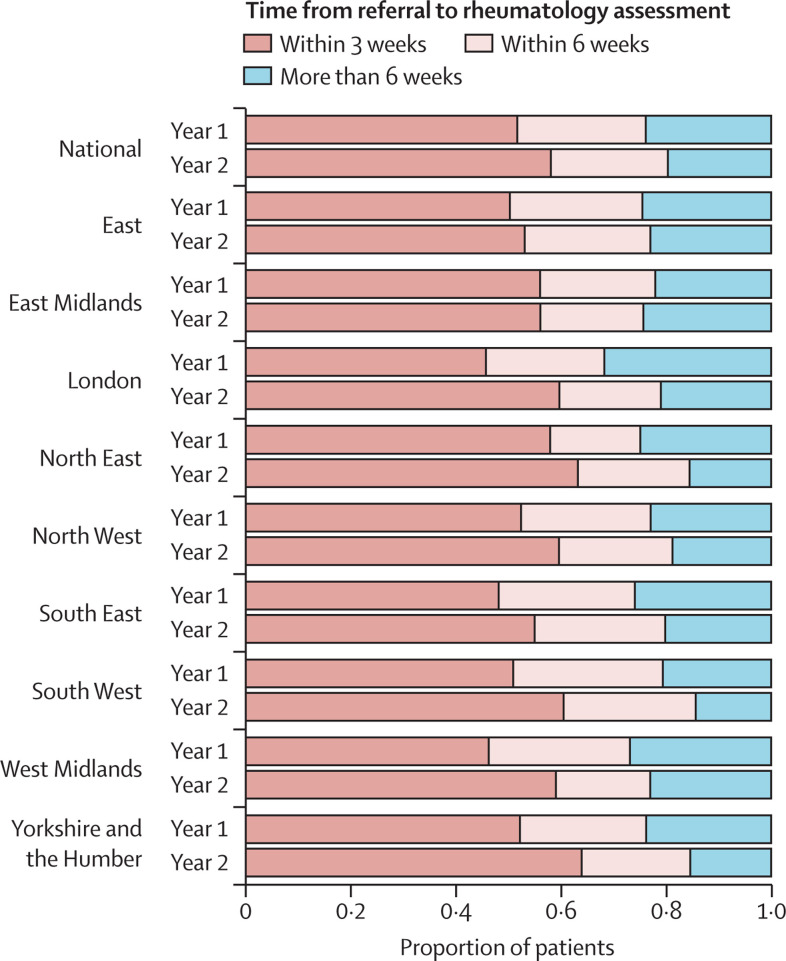
Rheumatology assessment times varied by region (figure 3 and appendix p 5). In the year before the pandemic, the North East of England had the highest proportion of patients assessed within 3 weeks (200 [58·0%] of 345), whereas London had the lowest proportion (170 [45·9%] of 370). Assessment times improved across all regions of England after April, 2020, albeit to varying degrees. Improvement was most apparent in London, where the proportion of patients assessed within 3 weeks increased from 170 (45·9%) of 370 to 140 (59·6%) of 235.
Figure 3.
Time from primary care referral to initial rheumatology assessment for people with incident inflammatory arthritis, overall and separated by region in England
The horizontal bars represent the mean proportion of patients with incident inflammatory arthritis who were assessed within 3, 6, or >6 weeks of referral. The year before the onset of the COVID-19 pandemic (Year 1: April 1, 2019, to March 31, 2020) and after (Year 2: April 1, 2020, to March 31, 2021) are compared for each region.
Data on the consultation medium used for initial rheumatology visits were available for 6675 (49·8%) of 13 405 patients. Of those with available data, the proportion of patients assessed via telephone or telemedicine increased from ten (0·3%) of 3425 before April, 2020, to 815 (25·1%) of 3250 in the year commencing April, 2020.
8625 (64·3%) of 13 405 patients were prescribed conventional synthetic DMARDs in primary care within 12 months of their first rheumatology appointment. Conventional synthetic DMARD prescribing varied by diagnosis (appendix p 6): 6665 (76·9%) of 8670 patients with incident rheumatoid arthritis were prescribed conventional synthetic DMARDs within 12 months, compared with 1625 (54·8%) of 2965 patients with psoriatic arthritis, 95 (7·5%) of 1275 patients with axial spondyloarthritis, and 240 (48·5%) of 495 patients with undifferentiated inflammatory arthritis. The median time from initial rheumatology assessment to prescription of a conventional synthetic DMARD in primary care was 92 days (IQR 42–172) for rheumatoid arthritis; 112 days (IQR 50–194) for psoriatic arthritis; 118 days (IQR 53–216) for axial spondyloarthritis, and 131 days (IQR 56–217) for undifferentiated inflammatory arthritis.
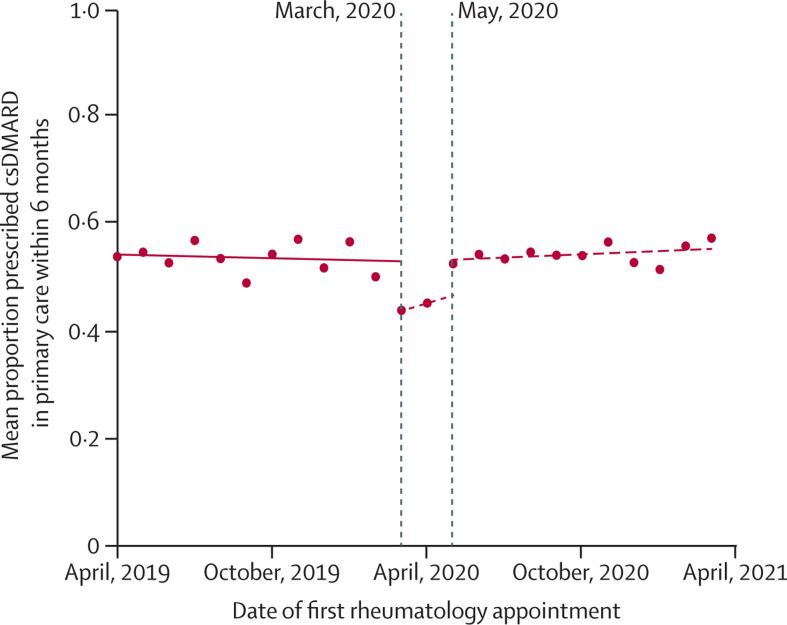
In interrupted time-series analysis models, the proportion of patients prescribed conventional synthetic DMARDs in primary care within 6 months of their first rheumatology appointment decreased from 275 (50·0%) of 550 to 230 (43·8%) of 525 between February, 2020, and March, 2020. This was followed by a return to prepandemic levels from May, 2020, onwards. Comparing prescribing trends before March, 2020 to after May, 2020, the observed trends were not significantly different (figure 4 ): trend pre-March, 2020: 0·014% reduction per year (95% CI –0·041 to 0·014); trend post-May, 2020: 0·025% improvement per year (95% CI –0·012 to 0·050); difference in trends, 0·038% per year (95% CI –0·0006 to 0·077; p=0·053).
Figure 4.
Interrupted time series analysis demonstrating trends in the proportion of patients with incident rheumatoid arthritis, psoriatic arthritis, or undifferentiated inflammatory arthritis who were prescribed csDMARDs in primary care within 6 months of initial rheumatology assessment
Single time point dots represent monthly averages. Trend lines are shown before March, 2020 (the onset of the first COVID-19 lockdown in England), and after May, 2020 (ie, after the two outlier months of March and April, 2020). A sensitivity analysis, including March and April, 2020, is shown in the appendix (p 10). csDMARD=conventional synthetic disease-modifying antirheumatic drug.
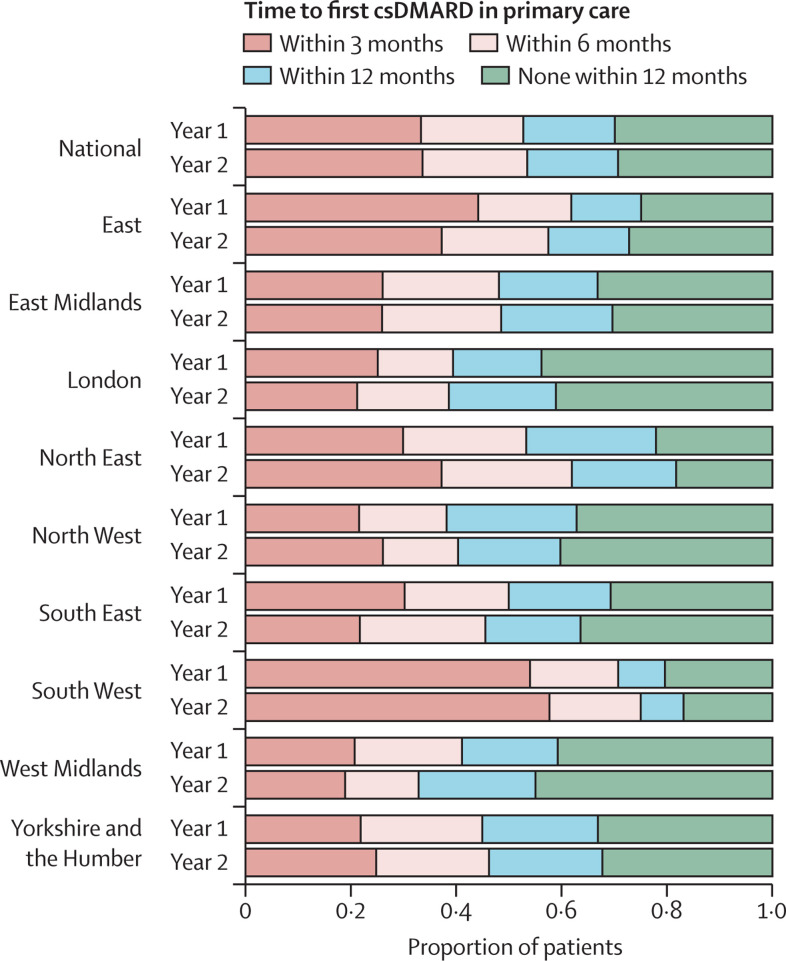
The proportion of patients with rheumatoid arthritis, psoriatic arthritis, or undifferentiated inflammatory arthritis prescribed conventional synthetic DMARDs in primary care varied markedly by region (figure 5 and appendix p 7). Before the pandemic, 515 (53·9%) of 955 patients in South West England were prescribed a conventional synthetic DMARD within 3 months of their first rheumatology appointment (760 [79·6%] of 955 within 12 months), compared with 135 (21·4%) of 630 patients in North West England (395 [62·7%] of 630 within 12 months). During the pandemic, there were no consistent changes in conventional synthetic DMARD prescribing nationally, with treatment delays improving in some regions and worsening in others.
Figure 5.
Time from initial rheumatology assessment to first prescription of a csDMARD in primary care for people with incident rheumatoid arthritis, psoriatic arthritis, or undifferentiated inflammatory arthritis; overall and separated by region in England
The horizontal bars represent the mean proportion of patients with incident inflammatory arthritis who were prescribed csDMARDs within 3, 6, and 12 months of their first rheumatology outpatient assessment. The year before the onset of the COVID-19 pandemic (Year 1: April 1, 2019, to March 31, 2020) and after (Year 2: April 1, 2020, to March 31, 2021) are compared for each region. csDMARD=conventional synthetic disease-modifying antirheumatic drug.
In the year before April, 2020, 2990 (63·5%) of 4705 first conventional synthetic DMARD prescriptions in primary care were for methotrexate, compared with 2175 (56·7%) of 3835 in the year after April, 2020; 60 (1·3%) of 4705 were for leflunomide before versus 40 (1·0%) of 3835 after April, 2020; 970 (20·6%) of 4705 were for hydroxychloroquine before versus 860 (22·4%) of 3835 after April, 2020; and 685 (14·6%) of 4705 were for sulfasalazine before versus 760 (19·8%) of 3835 after April, 2020.
Discussion
In this study, we have demonstrated the feasibility of using OpenSAFELY—a secure, population-level health dataset—to benchmark care quality for inflammatory arthritis. Our findings closely reflect those reported in the existing national audit of inflammatory arthritis care in England, without the need for manual data entry. We found that the number of recorded inflammatory arthritis diagnoses decreased by 40% early in the pandemic. For people who were referred, there was no evidence that rheumatology assessment times were affected by the pandemic. The proportion of patients prescribed conventional synthetic DMARDs in primary care was similar before and during the pandemic, with substantial underlying regional variation.
The 40% decrease in inflammatory arthritis diagnoses in the early pandemic is similar to what has been reported for other physical and mental health conditions.4 We observed subsequent decreases in inflammatory arthritis diagnoses that coincided with rising COVID-19 cases. With these decreases, the return to prepandemic levels occurred more quickly, suggesting the NHS and patient behaviour adapted as the pandemic progressed. Interestingly, no rebound in inflammatory arthritis diagnoses (ie, above prepandemic levels) was observed as of March, 2022, implying that a substantial burden of undiagnosed inflammatory arthritis remains as a consequence of the pandemic.
Rheumatology assessment times in our study closely match what has been reported in the existing national audit of inflammatory arthritis in England (NEIAA). In our study, between April, 2019, and April, 2020, 52% of patients were assessed by rheumatology within 3 weeks of referral; this compares with 48% of NEIAA patients during a similar timeframe. Regional variation in assessments times was also similar between our study and NEIAA.13
In contrast to NEIAA, for which mandatory data collection was paused, we could compare care before and during the pandemic. We found that rheumatology assessment times were shorter during the pandemic than before. The explanations for this are likely multifactorial. Even though services were under enormous strain during the pandemic, this might have been offset by fewer patients presenting with inflammatory arthritis and the transition to virtual consultations. Additionally, following the introduction of NEIAA in 2018, a national trend for improving rheumatology assessment times has been observed.13 Time to initial rheumatology assessment is benchmarked in NEIAA and tied to a best practice tariff paid to hospitals.25 We showed that these improvements continued despite the pandemic.
We observed disparities in care for people with different inflammatory arthritis diagnoses. Rheumatology assessment times were longer for patients with psoriatic arthritis or axial spondyloarthritis than for those with rheumatoid arthritis, while fewer patients with psoriatic arthritis or undifferentiated inflammatory arthritis were prescribed conventional synthetic DMARDs in primary care than patients with rheumatoid arthritis. Delays in diagnosis and treatment are well recognised problems in inflammatory arthritis, particularly for axial spondyloarthritis,26, 27 and they associate with greater disease progression.28 Our findings emphasise the importance of programmes to raise awareness about diagnostic delay,29 not only for axial spondyloarthritis but also for psoriatic arthritis.
During the pandemic, we found that the prescription of methotrexate and leflunomide as first conventional synthetic DMARDs decreased, whereas prescriptions for hydroxychloroquine and sulfasalazine increased. This might have reflected clinician concerns about prescribing medications perceived to be more immunosuppressive. Indeed, selective prescribing of less immunosuppressive medications in patients at risk of adverse outcomes might help to explain associations between sulfasalazine use and increased COVID-19 mortality.30 Conventional synthetic DMARDs with fewer monitoring requirements (eg, hydroxychloroquine) might also have been favoured during a time of limited access to blood tests and a shortage of blood test tubes in the NHS.31, 32
Our study had several strengths. Through use of routinely captured data in OpenSAFELY, we were able to benchmark care metrics in a very large population, with high reproducibility, and without the need for manual data collection. Use of routinely captured data reduces the potential for reporting bias, while increasing case ascertainment: in this study, we reported an incidence of rheumatoid arthritis of 3·9 per 10 000 adults between April, 2019, and April, 2020; in comparison, the number of patients with rheumatoid arthritis enrolled in NEIAA during a similar period equates to an incidence of 0·8 per 10 000 adults.13, 33 The scale and completeness of data in OpenSAFELY is greater than any other route for accessing primary care data in England. This provides an opportunity to expand national audits without demanding further resources from clinicians and with the capability to be updated in near real-time. As with all OpenSAFELY analyses, the complete set of code for the platform, for data curation, and for analysis is shared openly on GitHub for reuse under open licence.
Our study had limitations. As with other studies using coded EHR data, there is the potential for diagnostic misclassification, which could have overestimated inflammatory arthritis incidence. Code lists used in our analyses require validation in OpenSAFELY.34, 35, 36 Our adoption of the last primary care appointment before rheumatology assessment as a surrogate for referral date might have underestimated assessment delays (eg, if patients were reviewed again between referral and rheumatology assessment); however, similar findings between our study and NEIAA suggest this was of limited importance. We could only capture first rheumatology appointments for 65% of patients with incident inflammatory arthritis; this could relate to variations in coding of rheumatology appointments by hospital, or misclassification of patients with incident inflammatory arthritis. Additionally, prepandemic trends in our analyses reflect data from April, 2019 onwards, and might not be fully representative of earlier calendar years.
We were only able to capture primary care prescriptions for conventional synthetic DMARDs. As such, we cannot draw inferences on secondary care conventional synthetic DMARD prescribing. We have written extensively about the availability of hospital prescription data.37, 38 NHS Digital have recently made available prescription data for a subset of hospitals;39 we will seek to incorporate this in future work. We were unable to describe other important aspects of inflammatory arthritis care, such as disease education and patient-reported outcomes. Data on disease severity were also unavailable. We could not, therefore, determine whether disease phenotype affected care delivery (eg, prioritisation of cases with more severe disease). Together, this suggests that our methodology complements, but does not replace, existing national audits. Finally, although large, our study population might not be fully representative of the overall population of patients with inflammatory arthritis in England. OpenSAFELY-TPP covers approximately 40% of general practices in England, but only 17% of general practices in London. However, a recent study found that OpenSAFELY-TPP is largely representative of the English population in terms of IMD, age, sex, ethnicity, and causes of death.18
Previously, practical and privacy challenges around accessing routinely captured clinical data meant that national audits, such as NEIAA, have relied upon manual data collection by local teams. This imposes a substantial resource burden, as well as being challenging to reproduce on an ongoing basis. Using the OpenSAFELY framework, we were able to execute a single analysis for 40% of the population in near real-time while leaving the data in-situ, minimising reidentification risk. Our analyses can be extended to include OpenSAFELY-EMIS, thereby increasing data coverage to 99% of English general practices, as well as providing granular data on demographic and clinical subpopulations. Our approach can be applied to other diseases by making the OpenSAFELY framework available to NHS England, NICE, and HQIP. Finally, through close work with EHR software providers, and open reporting, OpenSAFELY can facilitate feedback to NHS organisations and front-line clinicians to improve clinical care.
In conclusion, during the early pandemic, there was a 40% reduction in recorded inflammatory arthritis diagnoses. No rebound increase in incidence above prepandemic levels was observed in later months, suggesting there remains a burden of undiagnosed inflammatory arthritis as a consequence of the pandemic. For people who sought medical attention, the impact of the pandemic on rheumatology assessment times and DMARD prescribing in primary care was less marked than might have been expected, and evidence of recovery was swift. Perhaps the most important message of this study, however, is that it is feasible to use routinely captured clinical data on a national scale to benchmark care quality for a long-term condition.
Data sharing
Access to the underlying identifiable and potentially reidentifiable pseudonymised electronic health record data is tightly governed by various legislative and regulatory frameworks and is restricted by best practice. The data in OpenSAFELY are drawn from general practice data across England where TPP is the data processor. TPP developers (Chris Bates, Jonathan Cockburn, John Parry, Frank Hester, and Sam Harper) initiate an automated process to create pseudonymised records in the core OpenSAFELY database, which are copies of key structured data tables in the identifiable records. These are linked onto key external data resources that have also been pseudonymised via SHA-512 one-way hashing of NHS numbers using a shared salt. DataLab developers and principal investigators (Ben Goldacre, Liam Smeeth, Caroline E Morton, Seb Bacon, Alex J Walker, William Hulme, Helen J Curtis, David Evans, Peter Inglesby, Simon Davy, George Hickman, Krishnan Bhaskaran, and Christopher T Rentsch) hold contracts with NHS England and have access to the OpenSAFELY pseudonymised data tables as needed to develop the OpenSAFELY tools. These tools in turn enable researchers with OpenSAFELY Data Access Agreements to write and execute code for data management and data analysis without direct access to the underlying raw pseudonymised patient data, and to review the outputs of this code. All code for the full data management pipeline—from raw data to completed results for this analysis—and for the OpenSAFELY platform as a whole is available for review at https://github.com/OpenSAFELY. The data management and analysis code for this paper was led by MDR and JBG.
This online publication has been corrected. The corrected version first appeared at thelancet.com/rheumatology on November 28, 2022
Declarations of interests
MDR has received honoraria from Lilly and Menarini, support for attending conferences from Lilly, Pfizer, Janssen, and UCB, and advisory board fees from Biogen. JBG has received honoraria from Abbvie, Biovitrum, Bristol Myers Squib (BMS), Celgene, Chugai, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Sobi, and UCB. BG has received research funding from the Laura and John Arnold Foundation, NIHR, NIHR School of Primary Care Research, NHS England, NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. AMa has received speaker fees from AbbVie and Galapagos, support for attending meetings from Lilly, and has participated in a technology appraisal for Filgotinib. AMe is a former employee and interim Chief Medical Officer of NHS Digital, and RCGP representative on GP Data Professional Advisory Group to NHS Digital. APC has received grants from BMS, consulting fees from BMS, AbbVie, and GSK/Galvini, speaker fees from BMS and AbbVie, and is on the executive committee of the EULAR research centre. SQ has received grant funding from BMS. JML is clinical director for NEIAA, secretary for The Federation of Joint Royal Colleges of Physicians Specialist Certificate Exam Board, and a trustee of the British Society for Rheumatology. AIR has received fees from Lilly for attending a conference. KB has received funding for the present study from Versus Arthritis and Pfizer Global Medical Grants for Quality Improvement in Rheumatology Practice. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
KB received funding from Versus Arthritis and Pfizer Global Medical Grants for Quality Improvement in Rheumatology Practice (68033839). MDR is funded by a National Institute for Health Research (NIHR) Doctoral Fellowship (NIHR300967). This research used data assets made available as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (MC_PC_20058). In addition, the OpenSAFELY Platform is supported by grants from the Wellcome Trust (222097/Z/20/Z); MRC (MR/V015757/1, MC_PC-20059, MR/W016729/1); NIHR (NIHR135559, COV-LT2-0073); and Health Data Research UK (HDRUK2021.000, 2021.0157). BG has also received funding from: the Bennett Foundation, the Wellcome Trust, NIHR Oxford Biomedical Research Centre, NIHR Applied Research Collaboration Oxford and Thames Valley, the Mohn-Westlake Foundation; all Bennett Institute staff are supported by BG's grants on this work. BMK is also employed by NHS England, working on medicines policy, and is clinical lead for primary care medicines data. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, Public Health England, or the Department of Health and Social Care. No funding bodies had any role in study design, data collection, analysis or interpretation, manuscript writing, or in the decision to submit the article for publication. We are very grateful for all the support received from the TPP Technical Operations team throughout this work, and for generous assistance from the information governance and database teams at NHS England and the NHS England Transformation Directorate. North East Commissioning Support Unit provided support on behalf of all Commissioning Support Units to aggregate the high-cost drugs data for use in OpenSAFELY studies.
Contributors
JBG, KB, MDR, BMK, BG, JML, and SN conceptualised the study. JBG, MDR, KB, CDA, BMK, AMa, APC, JML, SQ, EA, AIR, MAA, and SN developed the methodology. MDR, JBG, SN, and CDA conducted the formal analysis. MDR and JBG developed the diagnostic codelists. BG, BMK, BB-C, AMe, HJC, TO’D, and CDA developed the software. MDR wrote the original draft of the manuscript. All authors reviewed and edited the manuscript. All authors read and approved the final manuscript. JML was the senior sponsor of the study. MDR and JBG are the guarantors for the Article, and accept full responsibility for the work and the conduct of the study. MDR, JBG, and CDA had full access to all the data in the study, and accessed and verified the data. All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Coates LC, Conaghan PG, D'Agostino MA, et al. Remission in psoriatic arthritis—where are we now? Rheumatology (Oxford) 2018;57:1321–1331. doi: 10.1093/rheumatology/kex344. [DOI] [PubMed] [Google Scholar]
- 2.O'Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002;46:283–285. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- 3.Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavourable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397–1405. doi: 10.1007/s10067-014-2768-y. [DOI] [PubMed] [Google Scholar]
- 4.Williams R, Jenkins DA, Ashcroft DM, et al. Diagnosis of physical and mental health conditions in primary care during the COVID-19 pandemic: a retrospective cohort study. Lancet Public Health. 2020;5:e543–e550. doi: 10.1016/S2468-2667(20)30201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton J. Covid-19: A&E visits in England fall by 25% in week after lockdown. BMJ. 2020;369 doi: 10.1136/bmj.m1401. [DOI] [PubMed] [Google Scholar]
- 6.Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Health Foundation Use of primary care during the COVID-19 pandemic. 2020. https://www.health.org.uk/news-and-comment/charts-and-infographics/use-of-primary-care-during-the-covid-19-pandemic
- 8.British Medical Association The hidden impact of COVID-19 on patient care in the NHS in England. 2020. https://www.bma.org.uk/media/2841/the-hidden-impact-of-covid_web-pdf.pdf
- 9.Li J, Ringold S, Curtis JR, et al. Effects of the SARS-CoV-2 global pandemic on U.S. rheumatology outpatient care delivery and use of telemedicine: an analysis of data from the RISE registry. Rheumatol Int. 2021;41:1755–1761. doi: 10.1007/s00296-021-04960-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George MD, Danila MI, Watrous D, et al. Disruptions in rheumatology care and the rise of telehealth in response to the COVID-19 pandemic in a community practice-based network. Arthritis Care Res (Hoboken) 2021;73:1153–1161. doi: 10.1002/acr.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower H, Frisell T, di Giuseppe D, et al. Effects of the COVID-19 pandemic on patients with inflammatory joint diseases in Sweden: from infection severity to impact on care provision. RMD Open. 2021;7 doi: 10.1136/rmdopen-2021-001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healthcare Quality Improvement Partnership Measuring and improving our healthcare services. 2022. https://www.hqip.org.uk/about-us/
- 13.British Society for Rheumatology . Healthcare Quality Improvement Partnership; London: 2021. National Early Inflammatory Arthritis Audit (NEIAA) second annual report. [Google Scholar]
- 14.NICE Rheumatoid arthritis in over 16s. 2020. https://www.nice.org.uk/guidance/qs33
- 15.Ledingham JM, Yates M, Galloway JB. NEIAA: driving EIA service quality in a shifting clinical landscape. Rheumatology (Oxford) 2020;59:3127–3128. doi: 10.1093/rheumatology/keaa423. [DOI] [PubMed] [Google Scholar]
- 16.Yates M, Bechman K, Dennison EM, et al. Data quality predicts care quality: findings from a national clinical audit. Arthritis Res Ther. 2020;22:87. doi: 10.1186/s13075-020-02179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OpenSAFELY About OpenSAFELY. 2022. https://www.opensafely.org/about/
- 18.Andrews C, Schultze A, Curtis H, et al. OpenSAFELY: Representativeness of electronic health record platform OpenSAFELY-TPP data compared to the population of England. Wellcome Open Res. 2022;7:191. doi: 10.12688/wellcomeopenres.18010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NICE Rheumatoid arthritis in over 16s. 2013. https://www.nice.org.uk/guidance/qs33/documents/previous-version-of-quality-standard
- 20.NICE Spondyloarthritis in over 16s: diagnosis and management. 2017. https://www.nice.org.uk/guidance/ng65 [PubMed]
- 21.Ledingham J, Gullick N, Irving K, et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2017;56:865–868. doi: 10.1093/rheumatology/kew479. [DOI] [PubMed] [Google Scholar]
- 22.NHS Digital NHS Data model and dictionary. 2022. https://www.datadictionary.nhs.uk/attributes/treatment_function_code.html
- 23.Office for National Statistics NUTS level 1 (January 2018) full clipped boundaries in the United Kingdom. 2018. https://data.gov.uk/dataset/2aa6727d-c5f0-462a-a367-904c750bbb34/nuts-level-1-january-2018-full-clipped-boundaries-in-the-united-kingdom
- 24.Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15:480–500. [Google Scholar]
- 25.NHS England Consultation on 2021/22 National Tariff Payment System. Annex DtC: Guidance on best practice tariffs. 2021. https://www.england.nhs.uk/wp-content/uploads/2021/03/21-22NT_Annex-DtC-Best-practice-tariffs.pdf
- 26.Zhao SS, Pittam B, Harrison NL, Ahmed AE, Goodson NJ, Hughes DM. Diagnostic delay in axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2021;60:1620–1628. doi: 10.1093/rheumatology/keaa807. [DOI] [PubMed] [Google Scholar]
- 27.Russell MD, Coath F, Yates M, et al. Diagnostic delay is common for patients with axial spondyloarthritis: results from the National Early Inflammatory Arthritis Audit. Rheumatology (Oxford) 2022;61:734–742. doi: 10.1093/rheumatology/keab428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi E, Ahuja A, Rajput T, George AT, Park Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther. 2020;7:65–87. doi: 10.1007/s40744-020-00194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Axial Spondyloarthritis Society (NASS) Aspiring to Excellence. 2020. https://nass.co.uk/homepage/health-professionals/aspiring-to-excellence/
- 30.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher L, Hopcroft LEM, Rodgers S, et al. Changes in English medication safety indicators throughout the COVID-19 pandemic: a federated analysis of 57 million patients' primary care records in situ using OpenSAFELY. medRxiv. 2022 doi: 10.1101/2022.05.05.22273234. published online May 7. (preprint). [DOI] [Google Scholar]
- 32.Rimmer A. What has caused the NHS blood tube shortage, and how is it affecting doctors and patients? BMJ. 2021;374 doi: 10.1136/bmj.n2174. [DOI] [PubMed] [Google Scholar]
- 33.Office for National Statistics Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland
- 34.Muller S, Hider SL, Raza K, Stack RJ, Hayward RA, Mallen CD. An algorithm to identify rheumatoid arthritis in primary care: a Clinical Practice Research Datalink study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abhishek A, Doherty M, Kuo CF, Mallen CD, Zhang W, Grainge MJ. Rheumatoid arthritis is getting less frequent-results of a nationwide population-based cohort study. Rheumatology (Oxford) 2017;56:736–744. doi: 10.1093/rheumatology/kew468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abhishek A, Nakafero G, Kuo C-F, et al. Rheumatoid arthritis and excess mortality: down but not out. A primary care cohort study using data from Clinical Practice Research Datalink. Rheumatology (Oxford) 2018;57:977–981. doi: 10.1093/rheumatology/key013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowan A, Bates C, Hulme W, et al. A comprehensive high cost drugs dataset from the NHS in England—an OpenSAFELY-TPP Short Data Report. Wellcome Open Res. 2021;6:360. doi: 10.12688/wellcomeopenres.17360.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldacre B, MacKenna B. The NHS deserves better use of hospital medicines data. BMJ. 2020;370 doi: 10.1136/bmj.m2607. [DOI] [PubMed] [Google Scholar]
- 39.NHS Digital Electronic Prescribing and Medicines Administration (EPMA) data. 2022. https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/directions-and-data-provision-notices/data-provision-notices-dpns/electronic-prescribing-and-medicines-administration-data
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the underlying identifiable and potentially reidentifiable pseudonymised electronic health record data is tightly governed by various legislative and regulatory frameworks and is restricted by best practice. The data in OpenSAFELY are drawn from general practice data across England where TPP is the data processor. TPP developers (Chris Bates, Jonathan Cockburn, John Parry, Frank Hester, and Sam Harper) initiate an automated process to create pseudonymised records in the core OpenSAFELY database, which are copies of key structured data tables in the identifiable records. These are linked onto key external data resources that have also been pseudonymised via SHA-512 one-way hashing of NHS numbers using a shared salt. DataLab developers and principal investigators (Ben Goldacre, Liam Smeeth, Caroline E Morton, Seb Bacon, Alex J Walker, William Hulme, Helen J Curtis, David Evans, Peter Inglesby, Simon Davy, George Hickman, Krishnan Bhaskaran, and Christopher T Rentsch) hold contracts with NHS England and have access to the OpenSAFELY pseudonymised data tables as needed to develop the OpenSAFELY tools. These tools in turn enable researchers with OpenSAFELY Data Access Agreements to write and execute code for data management and data analysis without direct access to the underlying raw pseudonymised patient data, and to review the outputs of this code. All code for the full data management pipeline—from raw data to completed results for this analysis—and for the OpenSAFELY platform as a whole is available for review at https://github.com/OpenSAFELY. The data management and analysis code for this paper was led by MDR and JBG.
This online publication has been corrected. The corrected version first appeared at thelancet.com/rheumatology on November 28, 2022