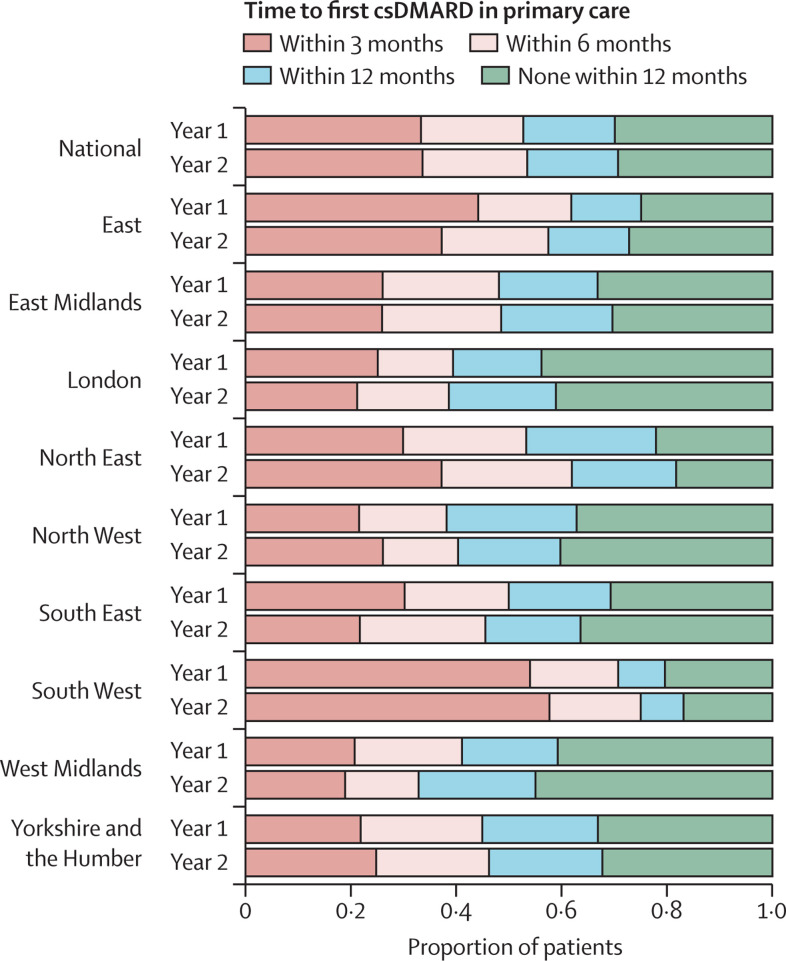
Figure 5.
Time from initial rheumatology assessment to first prescription of a csDMARD in primary care for people with incident rheumatoid arthritis, psoriatic arthritis, or undifferentiated inflammatory arthritis; overall and separated by region in England
The horizontal bars represent the mean proportion of patients with incident inflammatory arthritis who were prescribed csDMARDs within 3, 6, and 12 months of their first rheumatology outpatient assessment. The year before the onset of the COVID-19 pandemic (Year 1: April 1, 2019, to March 31, 2020) and after (Year 2: April 1, 2020, to March 31, 2021) are compared for each region. csDMARD=conventional synthetic disease-modifying antirheumatic drug.