Abstract
The COVID-19 pandemic has caused a global health crisis, and wastewater-based epidemiology (WBE) has emerged as an important tool to assist public health decision-making. Recent studies have shown that the SARS-CoV-2 RNA concentration in wastewater samples is a reliable indicator of the severity of the pandemic for large populations. However, few studies have established a strong correlation between the number of infected people and the viral concentration in wastewater due to variations in viral shedding over time, viral decay, infiltration, and inflow. Herein we present the relationship between the number of COVID-19-positive patients and the viral concentration in wastewater samples from three different hospitals (A, B, and C) in the city of Belo Horizonte, Minas Gerais, Brazil. A positive and strong correlation between wastewater SARS-CoV-2 concentration and the number of confirmed cases was observed for Hospital B for both regions of the N gene (R = 0.89 and 0.77 for N1 and N2, respectively), while samples from Hospitals A and C showed low and moderate correlations, respectively. Even though the effects of viral decay and infiltration were minimized in our study, the variability of viral shedding throughout the infection period and feces dilution due to water usage for different activities in the hospitals could have affected the viral concentrations. These effects were prominent in Hospital A, which had the smallest sewershed population size, and where no correlation between the number of defecations from COVID-19 patients and viral concentration in wastewater was observed. Although we could not determine trends in the number of infected patients through SARS-CoV-2 concentrations in hospitals' wastewater samples, our results suggest that wastewater monitoring can be efficient for the detection of infected individuals at a local level, complementing clinical data.
Keywords: Wastewater-based epidemiology, COVID-19 surveillance, COVID-19-positive patients, SARS-CoV-2 monitoring, Hospital wastewater
Graphical abstract
1. Introduction
Since the beginning of 2020, the world has been fighting one of its most challenging public health crises due to the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Thereafter, several strategies have been employed to monitor the spread of the disease. Classic epidemiology is based on clinical symptoms and diagnostic analyses. The quantitative polymerase chain reaction (RT-qPCR) assay was established as the golden standard to confirm COVID-19 cases around the world. However, the assay requires analytical instruments, skilled personnel, and long periods of data processing. Additionally, asymptomatic and presymptomatic cases often go unreported, indicating that the number of infections could be underestimated.
Wastewater-based epidemiology (WBE) has been used in the past few decades to assess the occurrence of pathogens at the community level (Li et al., 2012). Recently, it has been adopted to assess the spread of infectious diseases such as COVID-19 within communities based on variations of virus concentrations in time (Xagoraraki and O'Brien, 2020). In previous studies, viral shedding in the feces of COVID-19 patients has been observed (Wu et al., 2020), suggesting that WBE could be used as a biomarker for tracking community COVID-19 prevalence. WBE for SARS-CoV-2 surveillance has already been implemented in at least 58 countries across the world (Vogel, 2022), such as the United States (Gonzalez et al., 2020), Canada (D'Aoust et al., 2021), Argentina (Giraud-Billoud et al., 2021), Brazil (de Araújo et al., 2022; Mota et al., 2021), Spain (Randazzo et al., 2020), Germany (Westhaus et al., 2021), Italy (La Rosa et al., 2021), South Africa (Pillay et al., 2021), Qatar (Saththasivam et al., 2021), India (Kumar et al., 2020), Japan (Haramoto et al., 2020), and Australia (Ahmed et al., 2020a). Recent studies have shown that temporal changes in viral concentrations can indicate the potential onset of outbreaks within the sewershed (Galani et al., 2022; Zhu et al., 2021). For instance, Saguti et al. (2021) observed peaks of SARS-CoV-2 genome concentration 19 to 21 days prior to increases in the number of newly hospitalized patients in Gothenburg, Sweden.
Although the viral load in wastewater samples has been considered a reliable indicator of the severity of the pandemic (Fitzgerald et al., 2021; Rusiñol et al., 2021; Wurtzer et al., 2020), the prediction of the number of infected people based on the viral concentration or viral load has been challenging. Increases in the viral load have been associated with increases in the number of cases, hospitalizations, and deaths. However, few studies have established the relationship between the number of infected people and the viral load or concentration in the wastewater. For instance, Al-Faliti et al. (2022) explored the correlation between SARS-CoV-2 RNA load in wastewater and clinical case counts from 19 sewersheds across metropolitan areas in the U.S. The Spearman correlation coefficients (ρ) for raw data varied between 0.15 and 0.80. Weidhaas et al. (2021) reported a positive correlation between daily new COVID-19 case counts and SARS-CoV-2 gene copies per capita per day in wastewater samples (ρ = 0.54) combining the results of ten wastewater facilities in Utah, U.S. According to the authors, infiltration, inflow, virus decay and sewershed characteristics are important factors affecting the correlation analysis. Looking at a smaller scale, significant correlations were obtained between SARS-CoV-2 N1 and N2 gene concentrations in wastewater and case numbers in university dormitories in Utah, U.S (ρ = 0.55 and 0.56, respectively) (Lu et al., 2022).
A study conducted by Jiang et al. (2022) shows that several parameters are involved in the quantification of COVID-19 case numbers through WBE, such as viral RNA copies concentration in wastewater, viral shedding per gram of feces, daily shedding amount of feces per individual, in-sewer decay constant and in-sewer travel time. According to the authors, many WBE studies only capture a subset of these parameters. A susceptible-exposed-infectious-recovered (SEIR) model developed to estimate the number of people infected with COVID-19 in a sewershed was successfully calibrated using viral RNA copy rates in samples from a university's wastewater treatment plant and the number of confirmed COVID-19 cases among the university students through RT-PCR tests (McMahan et al., 2022). The authors affirmed that although their calibrated model might not generalize to other settings, it could be recalibrated if estimated daily case counts aligned with wastewater data are available.
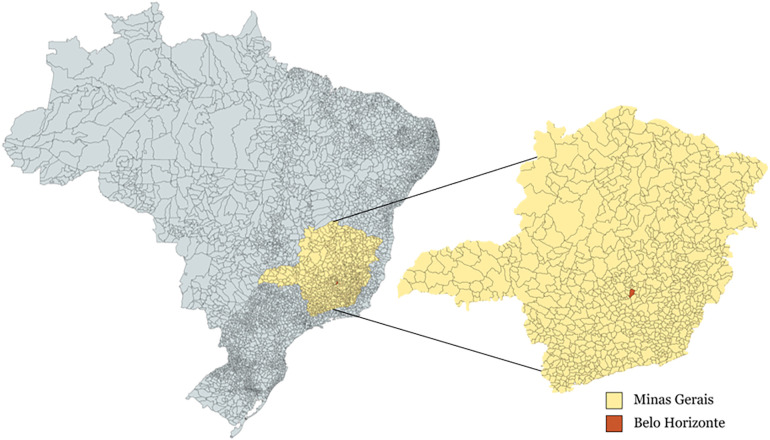
In this study, we aimed to establish a correlation between the number of infected patients in three different hospitals and the viral concentration measured in wastewater samples from these hospitals in the city of Belo Horizonte, Minas Gerais, Brazil (Fig. 1 ). In addition, our study aimed to compare the linear correlation coefficients obtained for the N1 and N2 regions of the SARS-CoV-2 nucleocapsid (N) gene, which are targets for the detection of the novel coronavirus (Centers for Disease Control and Prevention, 2020), and to assess the correlation between viral concentration and the number of defecations by COVID-19 patients during the sampling collection period. The results obtained in our study could help to evaluate predictions of the severity of the pandemic based on the viral load and concentration determined by qPCR analyses of wastewater samples for small populations.
Fig. 1.
Map showing the city of Belo Horizonte in the State of Minas Gerais, Brazil. Created with mapchart.net.
2. Material and methods
2.1. Wastewater sampling
We collected wastewater samples in the year 2020 from three different hospitals providing care to COVID-19 patients in the city of Belo Horizonte, Minas Gerais, Brazil (Fig. 2 ). The names of the hospitals are kept anonymous to comply with their confidentiality rules. For Hospital A, six composite samples were collected between May 19 and August 4. An automatic sampler was installed in a sewer manhole inside the hospital, receiving effluents primarily from inpatient units where COVID-19 patients were receiving treatment. For Hospital B, six samples were collected between May 25 and June 29. For Hospital C, seven samples were collected between May 29 and July 9. In those two hospitals, automatic samplers were placed in sewer manholes receiving contributions from all hospital buildings, prior to the connection to the public sewer. Composite samples were collected setting the samplers to store 400 mL every 10 min in a timespan between 4 and 6 h. The samples were collected in the morning, which is considered the peak time for feces concentration in wastewater. The samples were homogenized, and 1 L aliquots were transferred to 1-L bottles and taken to the Microbiology Laboratory of the Department of Sanitary and Environmental Engineering at the Federal University of Minas Gerais for the SARS-CoV-2 RNA quantification. The samples were kept at 4 °C during collection and transportation.
Fig. 2.
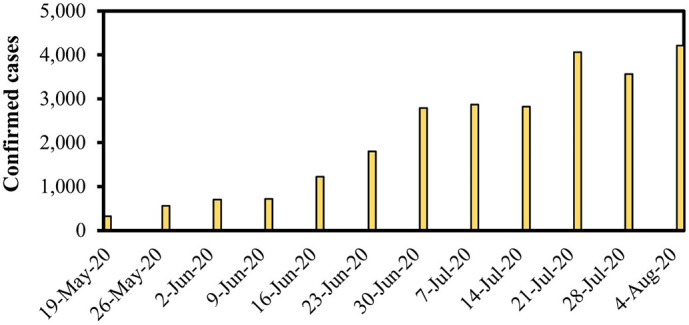
Number of confirmed cases of COVID-19 per week from May 19 to August 5, 2020, in the city of Belo Horizonte reported by the municipal administration (source: https://prefeitura.pbh.gov.br/saude/coronavirus).
2.2. Quantification of SARS-CoV-2 RNA
The SARS-CoV-2 RNA in the wastewater samples was quantified according to the methods described by Symonds et al. (2014) and Ahmed et al. (2015). The 1-L samples were homogenized and volumes of 100 mL were transferred to sterile Beckers in which a stock solution of MgCl2 (2.5 M) was added (1 mL/100 mL sample) and the pH was adjusted to 3.0–3.5 using acetic acid (1 M). Aliquots of 30 mL of the acidified samples were filtered using 0.45-μm electro-negative membranes (HAWP04700-Millipore, Massachusetts, USA). The membranes were kept in 2 mL tubes containing 600 μL of the PM1 solution from the DNA/RNA extraction kit, 6 μL of beta-mercaptoethanol, and glass beads, and stored at −80 °C prior to RNA extraction. RNA extractions were carried out according to the protocol of the manufacturer using AllPrep PowerViral DNA/RNA commercial kits (Qiagen, Hilden, Germany) using a bead beater to release viral particles attached to the membranes. The extracted nucleic acids were resuspended in 100 μL of RNase-free water and stored at −80 °C.
The quantification of the SARS-CoV-2 was performed through RT-qPCR targeting the N1 and N2 regions of the nucleocapsid gene using the primers recommended by the CDC (2020). The amplifications were carried out using iTaq Universal Probes One-Step kits (Biorad, California, USA) and reverse transcriptase (reaction volume of 20 μL). Plasmids purchased from Integrated DNA Technologies (Iowa, USA) were used as standards or positive controls. The assays were performed using an Applied Biosystems 7500 Real Time PCR System (Massachusetts, USA), and all the reactions were performed in triplicate. For each run, we included a series of three positive (2019-nCoV Positive Control from IDT, Iowa, USA) and negative (No Template Control, using sterile nuclease-free water) controls. An internal control was performed to verify PCR inhibition using primers/probes targeting the HsRPP30 gene, as recommended by the CDC. Whenever the sample did not amplify with the internal control (negative for RNAse P), it was diluted 10× and 100× to check for inhibition and confirm that the result was negative. Standard curves were built using serial dilutions of the standard plasmid containing the full sequence of the SARS-CoV-2 nucleocapsid (N) gene (IDT, Iowa, USA). The concentrations ranged from 1 × 10−5 to 1 gene copy/μL. The master standard curves used in this study were within the prescribed range of Minimum Information for Publication of Quantitative (MIQE) real-time PCR guidelines (Bustin et al., 2009). The amplification efficiencies for N1 assay ranged from 94.7 % to 109 %; R2 ranged from 0.993 to 0.997; slope ranged from −3.118 to −3.454 and Y-inter ranged from 35.726 to 38.667. The amplification efficiencies for N2 assay ranged from 90.2 % to 99 %; R2 ranged from 0.992 to 0.998; slope ranged from −3.327 to −3.582 and Y-inter ranged from 33.515 to 35.203. PCR triplicates with water were used as negative controls, in addition to the MERS-CoV Control and SARS-CoV Control obtained from IDT. A series of three negative and positive controls were included in each qPCR run. All qPCR assays were performed in triplicate according to the MIQE guidelines (Bustin et al., 2009). The limit of quantification (i.e. the lowest concentration where the dilution curve stops being linear) was 25 copies/reaction (or 10 gene copies/mL of sewage).
The limit of detection (LOD) was estimated according to an exponential model developed by Verbyla et al. (2016) using a 95 % probability of amplification, as described by Mota et al. (2021). The calculated LOD, considering 95 % probability of amplification, for this study was 9 copies/reaction or 3.6 copies/mL as detailed by de Araújo et al. (2022). In-sewer decay of the RT-qPCR signal for SARS-CoV-2 was considered negligible based on de Araújo et al. (2022).
2.3. Hospitals data
In Hospital A, the number of COVID-19 cases and the number of defecations from COVID-19 patients were monitored and reported by healthcare professionals during the days that wastewater samples were collected. Patients wearing diapers were not taken into account. The viral load was calculated by multiplying the viral concentration by the sampling time and the sewage flow rate. In Hospital B, the number of confirmed COVID-19 cases among COVID-19 hospitalized patients was reported by the hospital's administration. In Hospital C, the number of confirmed COVID-19 cases among hospitalized patients was obtained through daily bulletins available on the hospital's webpage. The Pearson correlation coefficient (R) was calculated to determine the relationship between the confirmed COVID-19 cases and the wastewater viral concentration in the three hospitals, and the number of defecations and viral concentration or viral load in wastewater samples from Hospital C. Correlations determined in this study were considered strong for R ≥ 0.68, moderate for 0.35 ≤ R < 0.68, and weak for R < 0.35 (Taylor, 1990).
3. Results and discussion
The study was performed from May 19 to August 5, 2020, during the 1st wave of the pandemic in Brazil, when the number of confirmed COVID-19 cases per week in the city of Belo Horizonte were exceeding 300, reaching a peak of 4210 on August 5. The circulation of multiple viral lineages was reported in Brazil in this period (such as B.1.1.28 and B.1.1.33), but variants of concern had not been reported (Giovanetti et al., 2022). According to a study from July 2020, the most common symptoms among hospitalized patients in Brazil were cough, fever, dyspnea, respiratory discomfort, and oxygen saturation below 95 % (de Souza et al., 2020). At the time, no vaccines against SARS-CoV-2 were available.
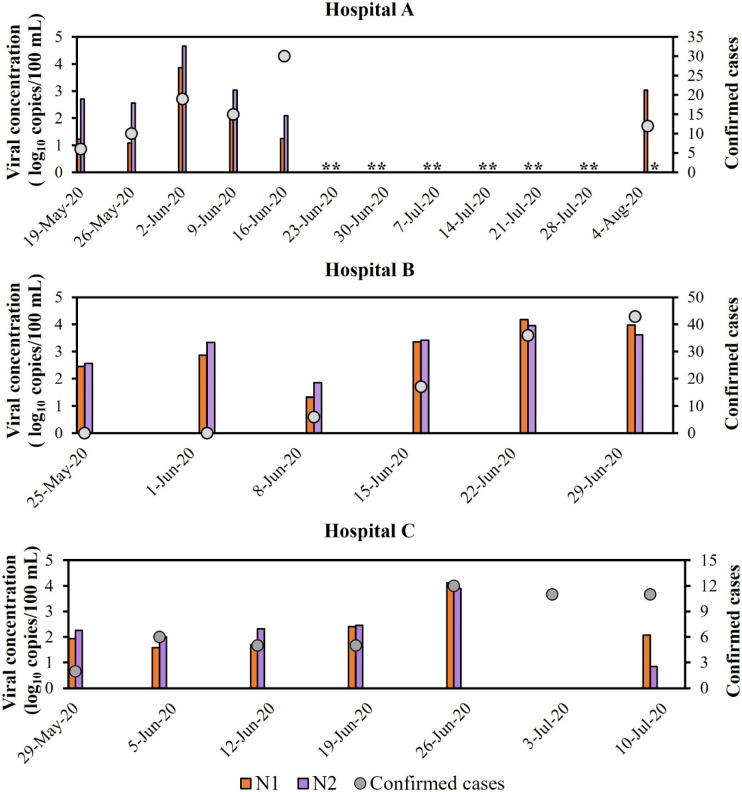
The SARS-CoV-2 genetic material concentrations were determined over time in wastewater samples from three different hospitals in the city of Belo Horizonte, Minas Gerais, Brazil. Fig. 3 indicates that the novel coronavirus was detected in all samples from the three hospitals, except the one collected on July 3, 2020 from Hospital C. The peak of COVID-19 confirmed cases in Hospital A was reported on June 16, when the lowest viral concentration was observed. However, the highest viral concentration in samples from this hospital was observed on June 2, 2020, two weeks before the confirmed cases peak. For Hospital B, higher viral concentrations were observed during the last two weeks of June 2020, when the highest numbers of confirmed cases were also reported. Nevertheless, incremental confirmed cases between those weeks were not followed by an increased viral concentration. Overall, the results followed the same upwards trend observed in the city of Belo Horizonte (Fig. 1). For Hospital C, the viral concentration peak on June 26, 2020 coincided with the highest number of confirmed cases reported during the monitoring period. However, the number of confirmed cases remained high during the following two weeks, while low viral concentrations were detected in the wastewater samples.
Fig. 3.
Number of confirmed cases of COVID-19 and viral concentration detected in wastewater samples (genes N1 and N2) from three different hospitals over time. Results for Hospital C on 3-Jul-2020 were negative, meaning that the sample did not amplify for CT < 40, according to CDC (2020). **No analysis performed. *No results reported for gene N2 on 5-Aug-2020.
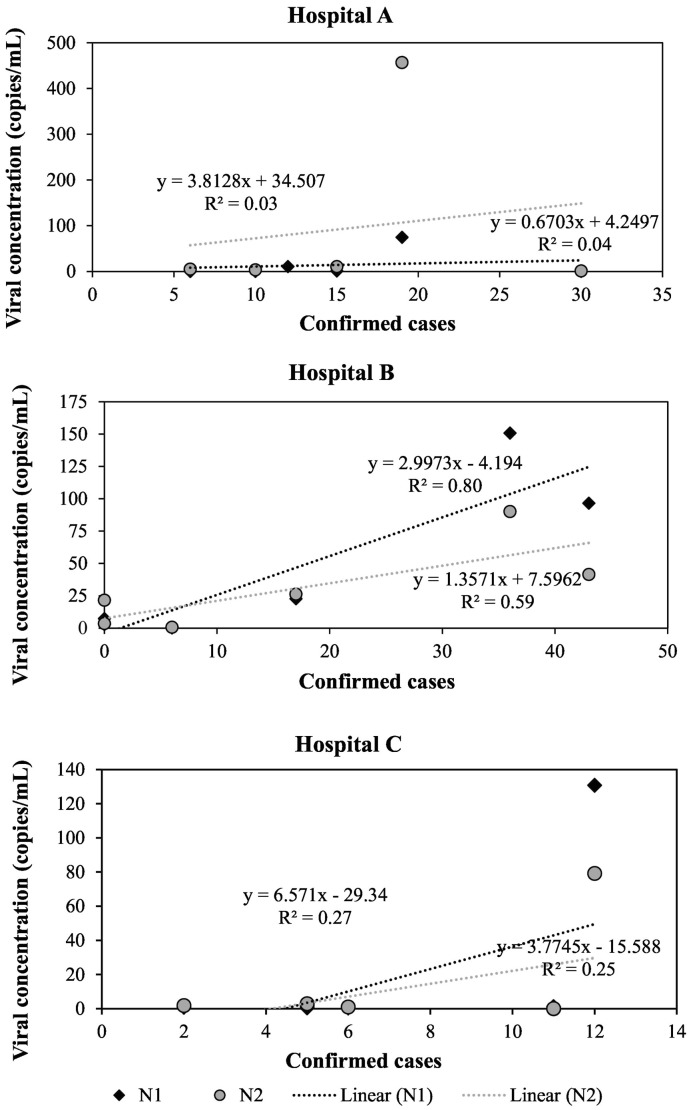
In 13 out of 18 wastewater samples collected in this study, the concentrations observed for the N2 region were higher than the concentrations observed for the N1 region of the nucleocapsid gene, while both regions led to positive results in all but one sample. Fig. 4 shows the correlations between viral concentrations and the number of confirmed cases for each hospital. In samples from Hospital A, very low correlation coefficients were observed (R = 0.19 and 0.18 for N1 and N2 regions, respectively). In samples from Hospital B, strong correlations between viral concentration and the number of confirmed cases were observed for both regions of the N gene (R = 0.89 and 0.77 for N1 and N2, respectively), while samples from Hospital C showed moderate correlations (R = 0.52 and 0.50 for N1 and N2, respectively).
Fig. 4.
Correlation between number of confirmed COVID-19 cases and viral concentration in wastewater samples from three different hospitals.
Our results show that wastewater monitoring is suitable to detect the presence of infected individuals at a local level. Nevertheless, the results suggest that the use of SARS-CoV-2 concentrations in hospitals' wastewater might not be appropriate to determine trends in the number of infected patients. Recent studies aimed to determine the concentrations of SARS-CoV-2 in the feces of infected patients and the duration of the shedding period. A shedding dynamics model fitted to data of hospitalized patients estimated that the medium viral concentration was 3.4 log copies/g-feces (95 % confidence interval: 0.24 to 6.5 log copies/g-feces) over the shedding period, with a concentration peak at 0.34 day (95 % confidence interval: 0.2 to 1.9 days). The medium estimated duration of viral shedding was 26.0 days (95 % confidence interval: 21.7 to 34.9 days) (Miura et al., 2021). This result was consistent with a previous clinical report, which indicated that for patients who tested positive for SARS-CoV-2 through respiratory swabs, the fecal samples remained positive for a mean of 27.9 days after the first symptom onset (Wu et al., 2020). Crank et al. (2022) suggested that viral load in excreta was the greatest source of variability in their model to assess the contribution of shedding routes to the load of SARS-CoV-2 RNA in wastewater. Additionally, a great impact on the calibration of a model to predict the number of infected individuals in a sewershed due to the maximum fecal shedding rate was observed by McMahan et al. (2022). Therefore, the variability of the viral concentration during the shedding period could be one of the reasons for the low correlation between viral concentrations and the number of confirmed COVID-19 cases, since patients at different stages of the infection could shed different amounts of the virus.
The viral concentrations of SARS-CoV-2 in wastewater samples could also be affected by the decay of the coronavirus in sewage pipes, which depends on the wastewater temperature and the matrix type (Ahmed et al., 2020b). Although the decay rate for the SARS-CoV-2 was found to be greater in untreated wastewater at higher temperatures, the average time required for a 1-log10 reduction of the viral concentration was calculated between 8.0 and 27.8 days, which is much higher than the sewage traveling times for the sampling points used in the present study, since our samples were collected at or very close to the hospitals. Also, a study conducted by McGain and Naylor (2014) shows that 20 to 40 % of the water within a hospital is used in wash basins, sinks, and showers, while 15 to 30 % is used in toilets. This data suggests that the fecal concentration in the hospital's wastewater is prone to high variability due to dilution with water used for several activities. For instance, Verlicchi et al. (2010) estimated that the water consumption in hospitals varies from 200 to 1200 L per hospital bed per day. Although our experimental design minimized the effects of viral decay and infiltration on the SARS-CoV-2 viral concentration, the water usage variability might have played a significant role in the dilution of our samples. This effect might have been more prominent in Hospital A, where samples were collected directly from an inpatient unit in the hospital, presumably more susceptible to water flow variability due to a smaller population size contributing to the wastewater samples. Higher level of variability for SARS-CoV-2 wastewater concentrations in smaller community sewershed areas was also observed by Holm et al. (2022) in a study comparing the abundance of SARS-CoV-2 as a function of urban sewershed size.
Disinfectants are typically used in large quantities in hospitals for surface and instrument cleaning and food processing. They contain mixtures of active ingredients such as alcohols, aldehydes, and chlorine-containing compounds (Verlicchi et al., 2010). However, contact time > 30 min and a high dosage of disinfectant are needed to achieve high virus inactivation rates (Achak et al., 2021). Acosta et al. (2021) presumed that interference of disinfectants and detergents in the hospital wastewater could lead to a loss of 3.6 % of viral genetic material in their SARS-COV-2 spiked controls. Due to the short sewage traveling times and short sample processing time in our study, we believe that disinfectants and detergents in the wastewater did not cause a significant loss of genetic material.
The concentration of SARS-CoV-2 in wastewater samples from hospitals has been assessed in other studies. A positive linear correlation between SARS-CoV-2 in the wastewater (gene N1) and prevalent COVID-19 cases was found for one out of three hospitals in the city of Calgary, in Canada, when the number of viral copies was normalized to the number of copies of the pepper mild mottle virus (PMMoV), which is used as a human fecal biomarker control, or when the Cq (PCR quantification cycle) value was used (Acosta et al., 2021). A study by Spurbeck et al. (2021) reported that the qPCR data for wastewater samples from a hospital in Toledo, in the U.S., correlated with the increasing number of COVID-19 hospitalizations over time, suggesting that a predictive model could be used to estimate the number of infected people over time. In addition, a study by Gonçalves et al. (2021) showed a comparison between the number of hospitalized COVID-19 patients and the concentration of SARS-CoV-2 copies in wastewater samples from a hospital in Ljubljana, Slovenia, targeting the envelope protein gene (E) and the RNA-dependent RNA polymerase gene (RdRP). The results showed that the SARS-CoV-2 RNA could be detected in the wastewater even when only one COVID patient was hospitalized. Considering studies on larger scales, de Freitas Bueno et al. (2022) reported correlation coefficients between the number of new COVID-19 cases and SARS-CoV-2 concentrations in wastewater ranging from 0.41 to 0.63 for four Brazilian cities. The authors indicated that few studies have reported strong correlations even for larger populations, with correlation coefficients reported in the literature ranging from 0.18 to 0.87.
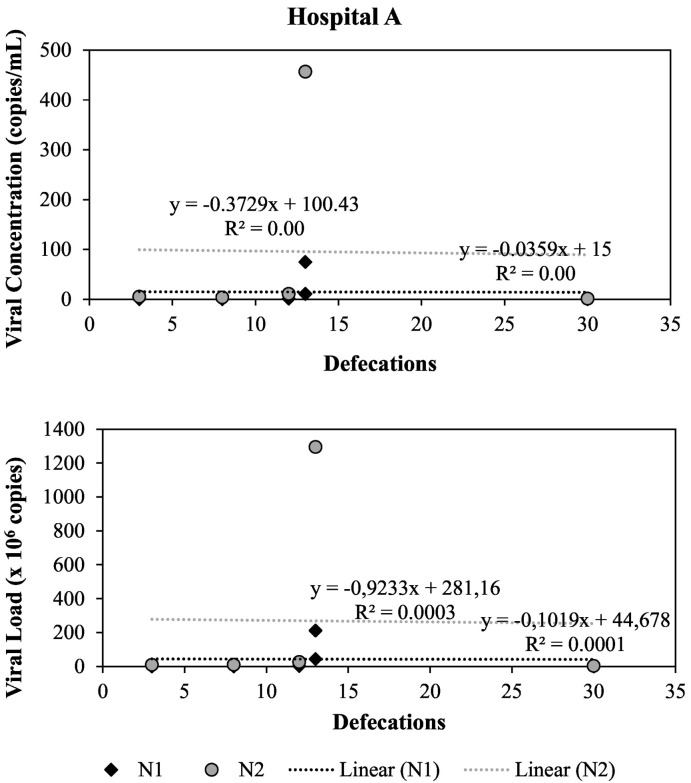
We also investigated the linear correlation between the number of times hospitalized COVID-19 patients defecated during the wastewater sampling period and the SARS-CoV-2 concentration or SARS-CoV-2 load for genes N1 and N2 in wastewater samples from Hospital A. As shown in Fig. 5 , our results indicated that there was no relationship between these variables in this study. As previously discussed, the lack of correlation could be due to viral dilution and high variability of viral concentration in feces from COVID-19 patients. For instance, Pedersen et al. (2022) showed that the SARS-CoV-2 RNA was detected in only 42 % of rectal swabs compared to 91 % for pharyngeal swabs from patients with preceding SARS-CoV-2 RT-qPCR positive samples.
Fig. 5.
Correlation between total number of defecations by COVID-19 patients and SARS-CoV-2 concentration and load in Hospital A.
4. Conclusions
Several studies have attributed the lack of strong correlations between the number of confirmed COVID-19 cases and the viral concentration in wastewater samples to viral decay, infiltration, inflow, and sewershed characteristics. In our study, we were able to reduce the effects of some of these parameters by minimizing the sewage traveling time and distance. We observed a strong correlation for Hospital B, a weak correlation for Hospital A, and a moderate correlation for Hospital C. No correlation was observed between the number of defecations from infected patients during the sampling period and viral concentration of load in the wastewater samples from Hospital A, suggesting that high variability of SARS-CoV-2 concentrations in feces of infected patients along the shedding period and feces dilution by fluctuating volumes of water used in the hospitals could have affected the viral concentrations in the samples. Despite the uncertainties regarding the use of viral concentrations in wastewater samples to determine the number of infected people in a specific location, our study confirms that wastewater-based epidemiology can provide early warnings of COVID-19 infections in sewersheds, as well as inform the presence of infected people in a building, as seen in other studies including universities buildings, dorms, and nursing homes.
CRediT authorship contribution statement
Juliana Calábria de Araújo: Conceptualization, methodology, writing – review, funding acquisition.
Camila L. Madeira: Data curation, writing – original draft.
Thiago Bressani: Conceptualization, sample acquisition, writing – review.
Cíntia Leal: Molecular analyses.
Deborah Leroy: Molecular analyses.
Elayne C. Machado: Molecular analyses.
Luyara A. Fernandes: Molecular analyses.
Maria Fernanda Espinosa: Molecular analyses.
Gabriel Tadeu O. Freitas: Sample acquisition.
Thiago Leão: Molecular analyses.
Vera Tainá Mota: Writing – review.
Alyne Duarte Pereira: Writing – review.
Carlos Perdigão: Conceptualization, writing – review.
Flávio Tröger: Conceptualization, writing – review.
Sérgio Ayrimoraes: Conceptualization, writing – review.
Marilia Carvalho de Melo: Conceptualization, funding acquisition.
Filipe Laguardia: Sample acquisition, writing – review.
Marcus Tulius Reis: Conceptualization, sample acquisition.
César Mota: Conceptualization, funding acquisition.
Carlos A. L. Chernicharo: Conceptualization, writing – review, funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the following institutions: National Water and Sanitation Agency (ANA), National Council for Scientific and Technological Development (CNPq), Minas Gerais State Agency for Research and Development (FAPEMIG), Coordination of Improvement of Higher Education Personnel (CAPES), and National Science and Technology Institute for Sustainable Sewage Treatment Plants (INCT Sustainable Sewage Treatment Plants).
Editor: Damià Barceló
Data availability
Data will be made available on request.
References
- Achak M., Alaoui Bakri S., Chhiti Y., M'hamdi Alaoui F.E., Barka N., Boumya W. SARS-CoV-2 in hospital wastewater during outbreak of COVID-19: a review on detection, survival and disinfection technologies. Sci. Total Environ. 2021;761 doi: 10.1016/J.SCITOTENV.2020.143192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta N., Bautista M.A., Hollman J., McCalder J., Beaudet A.B., Man L., Waddell B.J., Chen J., Li C., Kuzma D., Bhatnagar S., Leal J., Meddings J., Hu J., Cabaj J.L., Ruecker N.J., Naugler C., Pillai D.R., Achari G., Ryan M.C., Conly J.M., Frankowski K., Hubert C.R., Parkins M.D. A multicenter study investigating SARS-CoV-2 in tertiary-care hospital wastewater. Viral burden correlates with increasing hospitalized cases as well as hospital-associated transmissions and outbreaks. Water Res. 2021;201 doi: 10.1016/J.WATRES.2021.117369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/J.SCITOTENV.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191 doi: 10.1016/J.ENVRES.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Harwood V.J., Gyawali P., Sidhu J.P.S., Toze S. Comparison of concentration methods for quantitative detection of sewage-associated viral markers in environmental waters. Appl. Environ. Microbiol. 2015;81:2042–2049. doi: 10.1128/AEM.03851-14/ASSET/A5FDEC79-6952-47CF-B4AB-FF042EBE6455/ASSETS/GRAPHIC/ZAM9991160990003.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Faliti M., Kotlarz N., McCall C., Harris A.R., Smith A.L., Stadler L.B., Francis L., de los Reyes I., Vela J.D. Comparing rates of change in SARS-CoV-2 wastewater load and clinical cases in 19 sewersheds across four major metropolitan areas in the United States. ACS ES&T Water. 2022 doi: 10.1021/ACSESTWATER.2C00106. [DOI] [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. CDC 2019-Novel Coronavirus (2019-nCoV) Real-time RT-PCR Diagnostic Panel. [Google Scholar]
- Crank K., Chen W., Bivins A., Lowry S., Bibby K. Contribution of SARS-CoV-2 RNA shedding routes to RNA loads in wastewater. Sci. Total Environ. 2022;806 doi: 10.1016/J.SCITOTENV.2021.150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aoust P.M., Graber T.E., Mercier E., Montpetit D., Alexandrov I., Neault N., Baig A.T., Mayne J., Zhang X., Alain T., Servos M.R., Srikanthan N., MacKenzie M., Figeys D., Manuel D., Jüni P., MacKenzie A.E., Delatolla R. Catching a resurgence: increase in SARS-CoV-2 viral RNA identified in wastewater 48 h before COVID-19 clinical tests and 96 h before hospitalizations. Sci. Total Environ. 2021;770 doi: 10.1016/J.SCITOTENV.2021.145319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo J.C., Mota V.T., Teodoro A., Leal C., Leroy D., Madeira C., Machado E.C., Dias M.F., Souza C.C., Coelho G., Bressani T., Morandi T., Freitas G.T.O., Duarte A., Perdigão C., Tröger F., Ayrimoraes S., de Melo M.C., Laguardia F., Reis M.T.P., Mota C., Chernicharo C.A.L. Long-term monitoring of SARS-CoV-2 RNA in sewage samples from specific public places and STPs to track COVID-19 spread and identify potential hotspots. Sci. Total Environ. 2022;838 doi: 10.1016/J.SCITOTENV.2022.155959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Freitas Bueno R., Claro I.C.M., Augusto M.R., Duran A.F.A., Camillo L.de M.B., Cabral A.D., Sodré F.F., Brandão C.C.S., Vizzotto C.S., Silveira R., de Melo Mendes G., Arruda A.F., de Brito N.N., Machado B.A.S., Duarte G.R.M., de Lourdes Aguiar-Oliveira M. Wastewater-based epidemiology: a Brazilian SARS-COV-2 surveillance experience. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/J.JECE.2022.108298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza W.M., Buss L.F., Candido D.da S., Carrera J.P., Li S., Zarebski A.E., Pereira R.H.M., Prete C.A., de Souza-Santos A.A., Parag K.V., Belotti M.C.T.D., Vincenti-Gonzalez M.F., Messina J., da Silva Sales F.C., Nascimento V.H., Ghilardi F., Abade L., Gutierrez B., Andrade P.dos S., Kraemer M.U.G., Braga C.K.V., Aguiar R.S., Alexander N., Mayaud P., Brady O.J., Marcilio I., Gouveia N., Li G., Tami A., de Oliveira S.B., Porto V.B.G., Ganem F., de Almeida W.A.F., Fantinato F.F.S.T., Macário E.M., de Oliveira W.K., Nogueira M.L., Pybus O.G., Wu C.H., Croda J., Sabino E.C., Faria N.R. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat. Hum. Behav. 2020;4:856–865. doi: 10.1038/s41562-020-0928-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald S.F., Rossi G., Low A.S., McAteer S.P., O’Keefe B., Findlay D., Cameron G.J., Pollard P., Singleton P.T.R., Ponton G., Singer A.C., Farkas K., Jones D., Graham D.W., Quintela-Baluja M., Tait-Burkard C., Gally D.L., Kao R., Corbishley A. Site specific relationships between COVID-19 cases and SARS-CoV-2 viral load in wastewater treatment plant influent. Environ. Sci. Technol. 2021;55:15276–15286. doi: 10.1021/ACS.EST.1C05029. [DOI] [PubMed] [Google Scholar]
- Galani A., Aalizadeh R., Kostakis M., Markou A., Alygizakis N., Lytras T., Adamopoulos P.G., Peccia J., Thompson D.C., Kontou A., Karagiannidis A., Lianidou E.S., Avgeris M., Paraskevis D., Tsiodras S., Scorilas A., Vasiliou V., Dimopoulos M.A., Thomaidis N.S. SARS-CoV-2 wastewater surveillance data can predict hospitalizations and ICU admissions. Sci. Total Environ. 2022;804 doi: 10.1016/J.SCITOTENV.2021.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M., Slavov S.N., Fonseca V., Wilkinson E., Tegally H., Patané J.S.L., Viala V.L., San E.J., Rodrigues E.S., Santos E.V., Aburjaile F., Xavier J., Fritsch H., Adelino T.E.R., Pereira F., Leal A., de Carvalho Pereira G., Vazquez C., Sanabria G.M.E., Oliveira E.C.de, Iani F.C.de M., Demarchi L., Croda J., dos Santos Bezerra R., Paola Oliveira de Lima L., Martins A.J., Renata dos Santos Barros C., Marqueze E.C., de Souza Todao Bernardino J., Moretti D.B., Brassaloti R.A., Mariani P.D.S.C., Kitajima J.P., Santos B., Proto-Siqueira R., de Lello Rocha Campos Cassano R., Cantarelli V.V., Tosta S., Nardy V.B., Reboredo de Oliveira da Silva L., Gómez M.K.A., Lima J.G., Ribeiro A.A., Guimarães N.R., Watanabe L.T., Barbosa Da Silva L., da Silva Ferreira R., da Penha M.P.F., Ortega M.J., de la Fuente A.G., Villalba S., Torales J., Gamarra M.L., Aquino C., Figueredo G.P.M., Fava W.S., Motta-Castro A.R.C., Venturini J., do Vale Leone de Oliveira S.M., Gonçalves C.C.M., do Carmo Debur Rossa M., Becker G.N., Giacomini M.P., Marques N.Q., Riediger I.N., Raboni S., Mattoso G., Cataneo A.D., Zanluca C., Duarte dos Santos C.N., Assato P.A., Allan da Silva da Costa F., Poleti M.D., Lesbon J.C.C., Mattos E.C., Banho C.A., Sacchetto L., Moraes M.M., Grotto R.M.T., Souza-Neto J.A., Nogueira M.L., Fukumasu H., Coutinho L.L., Calado R.T., Neto R.M., Bispo de Filippis A.M., Venancio da Cunha R., Freitas C., Peterka C.R.L., de Fátima Rangel Fernandes C., Navegantes W., do Carmo Said R.F., Almiron M., Lourenço J., de Oliveira T., Holmes E.C., Haddad R., Campelo de A e Melo C.F., Sampaio S.C., Elias M.C., Kashima S., Junior de Alcantara L.C., Covas D.T. Genomic epidemiology of the SARS-CoV-2 epidemic in Brazil. Nat. Microbiol. 2022 doi: 10.1038/s41564-022-01191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud-Billoud M., Cuervo P., Altamirano J.C., Pizarro M., Aranibar J.N., Catapano A., Cuello H., Masachessi G., Vega I.A. Monitoring of SARS-CoV-2 RNA in wastewater as an epidemiological surveillance tool in Mendoza,Argentina. Sci. Total Environ. 2021;796 doi: 10.1016/J.SCITOTENV.2021.148887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J., Koritnik T., Mioč V., Trkov M., Bolješič M., Berginc N., Prosenc K., Kotar T., Paragi M. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci. Total Environ. 2021;755 doi: 10.1016/J.SCITOTENV.2020.143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., Thompson H., Keeling D., Mitchell J., Gonzalez D. COVID-19 surveillance in southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186 doi: 10.1016/J.WATRES.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/J.SCITOTENV.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm R.H., Mukherjee A., Rai J.P., Yeager R.A., Talley D., Rai S.N., Bhatnagar A., Smith T. SARS-CoV-2 RNA abundance in wastewater as a function of distinct urban sewershed size. Environ. Sci. Water Res. Technol. 2022;8:807–819. doi: 10.1039/D1EW00672J. [DOI] [Google Scholar]
- Jiang G., Wu J., Weidhaas J., Li X., Chen Y., Mueller J., Li J., Kumar M., Zhou X., Arora S., Haramoto E., Sherchan S., Orive G., Lertxundi U., Honda R., Kitajima M., Jackson G. Artificial neural network-based estimation of COVID-19 case numbers and effective reproduction rate using wastewater-based epidemiology. Water Res. 2022;218 doi: 10.1016/J.WATRES.2022.118451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/J.SCITOTENV.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Bonanno Ferraro G., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since december 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750 doi: 10.1016/J.SCITOTENV.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Xiao L., Wang L., Zhao S., Zhao X., Duan L., Guo M., Liu L., Feng Y. Molecular surveillance of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi by genotyping and subtyping parasites in wastewater. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu E., Ai Y., Davis A., Straathof J., Halloran K., Hull N., Winston R., Weir M.H., Soller J., Bohrerova Z., Oglesbee M., Lee J. Wastewater surveillance of SARS-CoV-2 in dormitories as a part of comprehensive university campus COVID-19 monitoring. Environ. Res. 2022;212 doi: 10.1016/J.ENVRES.2022.113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGain F., Naylor C. Environmental sustainability in hospitals – a systematic review and research agenda. J. Health Serv. Res. Policy. 2014;19:245–252. doi: 10.1177/1355819614534836. [DOI] [PubMed] [Google Scholar]
- McMahan C.S., Lewis D., Deaver J.A., Dean D., Rennert L., Kalbaugh C.A., Shi L., Kriebel D., Graves D., Popat S.C., Karanfil T., Freedman D.L. Predicting COVID-19 infected individuals in a defined population from wastewater RNA data. ACS EST Water. 2022 doi: 10.1021/ACSESTWATER.2C00105/ASSET/IMAGES/LARGE/EW2C00105_0003.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura F., Kitajima M., Omori R. Duration of SARS-CoV-2 viral shedding in faeces as a parameter for wastewater-based epidemiology: re-analysis of patient data using a shedding dynamics model. Sci. Total Environ. 2021;769 doi: 10.1016/J.SCITOTENV.2020.144549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota C.R., Bressani-Ribeiro T., Araújo J.C., Leal C.D., Leroy-Freitas D., Machado E.C., Espinosa M.F., Fernandes L., Leão T.L., Chamhum-Silva L., Azevedo L., Morandi T., Freitas G.T.O., Costa M.S., Carvalho B.O., Reis M.T.P., Melo M.C., Ayrimoraes S.R., Chernicharo C.A.L. Assessing spatial distribution of COVID-19 prevalence in Brazil using decentralised sewage monitoring. Water Res. 2021;202 doi: 10.1016/J.WATRES.2021.117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen R.M., Tornby D.S., Bang L.L., Madsen L.W., Skov M.N., Sydenham T.V., Steinke K., Jensen T.G., Johansen I.S., Andersen T.E. Rectally shed SARS-CoV-2 in COVID-19 inpatients is consistently lower than respiratory shedding and lacks infectivity. Clin. Microbiol. Infect. 2022;28:304.e1–304.e3. doi: 10.1016/J.CMI.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay L., Amoah I.D., Deepnarain N., Pillay K., Awolusi O.O., Kumari S., Bux F. Monitoring changes in COVID-19 infection using wastewater-based epidemiology: a South African perspective. Sci. Total Environ. 2021;786 doi: 10.1016/J.SCITOTENV.2021.147273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/J.WATRES.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiñol M., Zammit I., Itarte M., Forés E., Martínez-Puchol S., Girones R., Borrego C., Corominas L., Bofill-Mas S. Monitoring waves of the COVID-19 pandemic: inferences from WWTPs of different sizes. Sci. Total Environ. 2021;787 doi: 10.1016/J.SCITOTENV.2021.147463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguti F., Magnil E., Enache L., Churqui M.P., Johansson A., Lumley D., Davidsson F., Dotevall L., Mattsson A., Trybala E., Lagging M., Lindh M., Gisslén M., Brezicka T., Nyström K., Norder H. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res. 2021;189 doi: 10.1016/J.WATRES.2020.116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saththasivam J., El-Malah S.S., Gomez T.A., Jabbar K.A., Remanan R., Krishnankutty A.K., Ogunbiyi O., Rasool K., Ashhab S., Rashkeev S., Bensaad M., Ahmed A.A., Mohamoud Y.A., Malek J.A., Abu Raddad L.J., Jeremijenko A., Abu Halaweh H.A., Lawler J., Mahmoud K.A. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021;774 doi: 10.1016/J.SCITOTENV.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck R.R., Minard-Smith A., Catlin L. Feasibility of neighborhood and building scale wastewater-based genomic epidemiology for pathogen surveillance. Sci. Total Environ. 2021;789 doi: 10.1016/J.SCITOTENV.2021.147829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds E.M., Verbyla M.E., Lukasik J.O., Kafle R.C., Breitbart M., Mihelcic J.R. A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Res. 2014;65:257–270. doi: 10.1016/J.WATRES.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Taylor R. Interpretation of the correlation coefficient: a basic review. J. Diagn. Med. Sonogr. 1990;6:35–39. doi: 10.1177/875647939000600106. [DOI] [Google Scholar]
- Verbyla M.E., Symonds E.M., Kafle R.C., Cairns M.R., Iriarte M., Mercado Guzmán A., Coronado O., Breitbart M., Ledo C., Mihelcic J.R. Managing microbial risks from indirect wastewater reuse for irrigation in urbanizing watersheds. Environ. Sci. Technol. 2016;50:6803–6813. doi: 10.1021/ACS.EST.5B05398/SUPPL_FILE/ES5B05398_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Verlicchi P., Galletti A., Petrovic M., BarcelÓ D. Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J. Hydrol. 2010;389:416–428. doi: 10.1016/J.JHYDROL.2010.06.005. [DOI] [Google Scholar]
- Vogel G. Signals from the sewer. Science. 2022;375:1100–1104. doi: 10.1126/SCIENCE.ADB1874. [DOI] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K., Laan J.Vander, LaCross N. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/J.SCITOTENV.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/J.SCITOTENV.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance. 2020;25:1. doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xagoraraki I., O'Brien E. Wastewater-Based Epidemiology for Early Detection of Viral Outbreaks. 2020. pp. 75–97. [DOI] [Google Scholar]
- Zhu Y., Oishi W., Maruo C., Saito M., Chen R., Kitajima M., Sano D. Early warning of COVID-19 via wastewater-based epidemiology: potential and bottlenecks. Sci. Total Environ. 2021;767 doi: 10.1016/J.SCITOTENV.2021.145124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.