To the Editor,
Two recent Letters to Editor,1 , 2 each including a meta-analysis, argued in favor of efficacy of early commenced fluvoxamine treatment in prevention of disease progression in (mild) COVID-19 (out)patients. There are, however, several points common to both of them that need to be addressed. First, both meta-analyses reported effect estimates pooled across several randomized (placebo) controlled trials (RCTs) but including also two non-randomized, open-label studies in which patients opted to take fluvoxamine or not. These two studies, heavily burdened by sampling/selection bias, contributed a considerable part of the total amount of data.1 , 2 Both meta-analyses1 , 2 used the reported raw (unadjusted) proportions from these studies and combined them with RCT data. Under certain very strict conditions, non-randomized studies might be included in meta-analysis of RCTs,3 but treating non-randomized data as if they were generated in RCTs is bluntly inappropriate.3 Next, both meta-analyses used random-effects pooling combining some very small studies with only a few or no events and some rather large studies (with around 10-fold difference in size between them).1 , 2 While this is not an uncommon practice, it generates a problem in estimation of the across-study variance (τ2)4. Namely, and particularly in the case of binary outcomes, small studies (particularly with no or only a few events), are more heterogeneous than the large(r) ones, and variance estimates are much more imprecise.4 When small and large studies are combined, only one variance estimate is generated which, clearly, does not really fit either of them – it understimates true heterogeneity in small trials, and overestimates it in larger trials – but it is used to assign study weights, and this affects pooled point-estimates and confidence intervals.4 Finally, both meta-analyses largely focused on hospitalizations as the outcome. Indeed, in this setting, incident hospitalization is a reasonable indicator of disease worsening. However, it is not the only indicator – there are other clinical events that are comparably as informative and are complementary to hospitalizations. For example, any event prompting emergency room visit or other forms of urgent help also illustrates disease progression. If such events are disregarded, one may not only fail to get a full picture of the reality, but this could also bias the estimates related to hospitalization. It is not inconceivable that, for example, a patient requiring urgent help that can be provided during an emergency room visit could benefit from this help in a way that will allow him/her to avoid (imminent) hospitalization. In such a scenario, treatment that results in more such vistis than another one (and is, hence, inferior in terms of preventing disease worsening) might turn out to be comparable or superior regarding hospitalizations (since avoided due to preceding events).
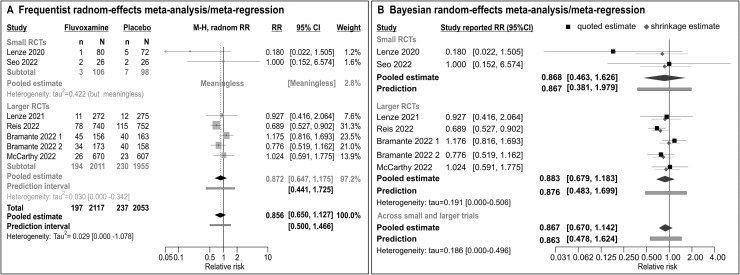
Table 1 contains all RCTs (cumulatively) included in the two1 , 2 meta-analyses: most of them used composite outcomes to adequately illustrate disease progression. However, Table 1 differs from data used in the published meta-analyses 1 , 2 in that: (i) it includes only RCTs (placebo-controlled, double-blind) and no non-randomized studies; (ii) it includes one small RCT conducted in South Korea at the very beginning of the pandemics, althouth only recently published (depicted as “Seo” in Table 1), that was not included – and should have been – in either of the published meta-analyses1 , 2; (iii) it indicates that one larger trial (depicted as “Bramante” in Table 1) actually consisted of two fluvoxamine “subtrials” (fluvoxamine+metformin was compared to placebo+metformin, or fluxoxamine+placebo was compared to “double placebo”) that yielded estimates in opposite directions. Finally, Table 1 depicts the outcome more comprehensively illustrative of “disease progression” than just “hospitalization”, used to generate meta-analysis in Fig. 1 . Frequentist meta-analysis is based on (random-effects) regression approach to generate estimates of the treatment effect and of heterogeneity at each level of “study size” as a binary moderator (Fig. 1A): (i) two small trials are so different in reported estimates (indicating a “huge” effect or no effect), that a pooled estimate is meaningless. Estimated variance is huge, but meaningless since likely imprecise with no possibility to generate its confidence intervals; (ii) considering large(r) trials, heterogeneity is lower, but still considerable (prediction intervals extend from 56% lower to 72.5% higher relative risk with fluvoxamine), and pooled estimate does not indicate any relevant benefit of fluvoxamine (RR=0.872, 0.647–1.175); (iii) a single estimate across all small and larger trials is also reported (note extremely wide confidence intervals around the estimated τ2) to allow for a comparison of the present and published 1 , 2 analyses: it also does not indicate any relevant benefit of fluvoxamine (RR=0.856, 95% CI 0.650–1.127) with prediction intervals extending form 50% lower to 46.6% higher (relatively) risk with fluvoxamine than with placebo. Bayesian random-effects meta-analysis/meta-regression (based on quite different computational background) yields similar results (Fig. 1B): (i) no indication of a benefit based on small RCTs (RR=0.868, 95%HPD CrI 0.463–1.626), with very wide prediction interval (from 2.5 lower to 98% higher relative risk of the outcome with fluvoxamine); (ii) no indication of a benefit based on large RCTs (RR=0.883, 0.679–1.182) with prediction extended from twice lower to 70% higher (relatively) risk with fluvoxamine); (iii) and no indication of a benefit in a random-effect meta-analysis across all trials (RR=0.867, 0.67–1.142), again with wide prediction interval (Fig. 1B).
Table 1.
Randomized placebo-controlled trials (all parallel-group, double-blind) of fluvoxamine in COVID-19 outpatients included in the present analysis.
| Author | Population | Fluvoxamine | Control | Reported outcome | For the present analysis | Source of outcome data |
|---|---|---|---|---|---|---|
| Lenze 20205USA | Adult, not vaccinated, PCR-positive, ≤7 days since the symptom onset, ≥92% oxygenation on room air; free of severe comorbidities /immune suppression. | Single 50 mg dose; then 2 × 100 mg over 2 days and up-titrated to 3 × 100 mg up to 15 days (if tolerated) | Matching placebo | New-onset dyspnea or hospitalization for dyspnea or pneumonia + saturation drop to <92% over 15 days | Hospitalization for dyspnea or pneumonia with saturation drop over 15 days | Updated trial data provided in a review by Lee et al.6 (Figure 2) |
| Lenze 20217USA | Age ≥30 years, confirmed COVID-19, not vaccinated, mild symptoms, ≥92% oxygenation on room air; free of severe comorbidities /immune suppression | Up to 2 × 100 mg, 15 days (as tolerated) | Matching placebo | New-onset dyspnea or hospitalization for dyspnea or pneumonia + saturation drop to <92% over 15 days | Hospitalization for dyspnea or pneumonia with saturation drop over 15 days | Updated trial data provided in a review by Lee et al.6 (Figure 2) |
| Seo 20228South Korea | Adult, PCR-positive, ≤7 days since the symptom onset, mild symptoms; free of severe comorbidities /immune suppression. | Single 50 mg dose; then 2 × 100 mg, 10 days (as tolerated) | Matching placebo | Saturation drop to <94% or new onset pneumonia/dyspnea with infiltrate on chest X-ray over 10 days | Reported outcome | Published study (text) |
| Reis 20229Brazil | Adult, confirmed COVID-19, ≤7 days since the symptom onset, not vaccinated, mild symptoms + at least one factor suggestive of a high-risk patient | 2 × 100 mg, 15 days | Matching placebo | Hospitalization or emergency room visit due to COVID-19 that is >6 h duration over 28 days | Reported outcome | Published study (Table 2) |
| Bramante 202210USA | Age 30–85 years, overweight-obese, confirmed COVID-19, mild sysmptoms, ≤7 days since the symptom onset + renal or liver or cardiovascular condition associated with a high risk, but not unstable, and not immunocompromised | 1. Fluvoxamine 2 × 50 mg + placebo, 14 days 2. Fluvoxamine 2 × 50 mg + metformin, 14 days |
1. “Double” matching placebo 2. Matching placebo + metformin |
Oxygenation drop to ≤93% or emergency department visit or hospitalization or death over 14 days | Oxygenation drop or emergency department visit or hospitalization over 14 days(no patient died) | Published study – online supplement, Figure S1C |
| McCarthy 202211USA | Age ≥30 years, confirmed COVID-19, ≤7 days since the symptom onset, mild symptoms | 2 × 50 mg, 10 days | Matching placebo | Hospitalization, urgent care, emergency room visit or death over 28 days | Hospitalization, urgent care or emergency room visit over 28 days (no patient died) | Published study (Table 2) |
Fig. 1.
Meta-analysis of randomized placebo-controlled trials of fluvoxamine outlined in Table 1. Please note, “Bramante 2022 1″ refers to a comparison of fluvoxamine + metformin placebo vs. “double” matching placebo, while “Bramante 2022 2″ refers to the comparison of fluvoxamine + metformin vs. fluvoxamine placebo + metformin. Since both comparisons come from the same trial, usually the “overall” estimate of fluvoxamine vs. placebo is referred to. However, as shown here, the two comparisons yielded estimates in opposing directions (albeit, imprecise): by disregarding this discrepancy (as small as it might be) and using the “raw overall estimate”, one artificially reduces heterogeneity across fluvoxamine vs. placebo comparisons. A Frequentist random effects meta-analysis/meta-regression addressed “study size” as a categorical moderator and yielded effect and heterogeneity estimates at each level of the moderator, as well as the overall one (restricted maximum likelihood estimator of τ2, with Hartung-Knapp-Sidik-Jonkman adjustment to t-distribution). B Bayesian random-effects meta-analysis/meta-regression used the same approach, but under the Bayesian framework, with weakly informative prior for τ2 (half Cahuchy, scale=0.5), and moderately informative skeptical prior for the pooled estimate compatible with the a priori hypothesis of no treatment effect [normal (0.0, 0.355) for ln(RR) – assigns 50% probability to RR <1.0, and 50% probability to RR >1.0].
Overall, the two published meta-analyses 1 , 2 adopted a choice of the outcome that might not be fully illustrative for the intended purpose, which combined with some methodological drawbacks resulted in estimates that are likely inaccurate, i.e., overtly optimistic. Based on the present analysis, it seems reasonable to conclude that the current best available evidence rather convincingly demonstrates that fluvoxamine – at dosing regimens otherwise viewed as acceptably safe (as one would expect in drug repurposing efforts) - conveys no relevant benefit in this setting.
Funding
This work received no funding.
Data availability
All data used in this work are presented in the manuscript (Table 1, Fig. 1)
Declaration of Competing Interest
I have no financial or non-financial conflict of interest to declare.
References
- 1.Cheema H.A., Jafar U., Elrashedy A.A., Shahid A., Awan R.U., Ehsan M., et al. Efficacy and safety of fluvoxamine for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Infect. 2022 doi: 10.1016/j.jinf.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marčec R., Dodig V.M., Likić R. A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients. TOGETHER making a difference. J Infect. 2022 doi: 10.1016/j.jinf.2022.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves B.C., Deeks J.J., Jiggins J.P.T., Shea B., Tugwell P., Wells G.A, et al. In: Cochrane Handbook of Systematic Reviews of Interventions v. 6.2 (updated February 2021) Cochrane. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Avaialbe at Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training; 2021. Chapter 24: including non-randomized studies on intervention effects. [Google Scholar]
- 4.IntHout J., Ioannidis J.P.A., Borm G.F., Goeman J. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epicemiol. 2015;68:860–869. doi: 10.1016/j.jclinepi.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweigher J., Nicol G.E., et al. Fluvoxamine vs. placebo and clinical deterioration in outpatients with symptomatic COVID-19. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee T.C., Vigod S., Bortolussi-Courval E., Hanula R., Boulware D.R., Lenze E.J., et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization. A systematic review and meta-analysis. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenze E. Fluvoxamine for early treatment of covid-19: a fully-remote, randomized placebo controlled trial. ClinicalTrials.gov. 2022 https://clinicaltrials.gov/ct2/show/NCT04668950 Accessed November 15. [Google Scholar]
- 8.Seo H., Kim H., Bae S., Park S., Chung H., Sung HS, et al . Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother. 2022;54:102–113. doi: 10.3947/ic.2021.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis G., dos Santos-Moreira-Silva A., Medeiros Silva D.C., Thabane L., Cruz Milagres A.,., Santiago Ferreira T., et al. Effect of early treatment with fluvoxamine on riks of emergeny care and hospitalization among patients with COVID-19: the TOGETHER randomized, platform clinical trial. Lancet Glob Health. 2022;10:e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramante C.T., Huling J.D., Tignanelli C.J., Buse J.B., Liebovitz D.M., Nicklas J.M., et al. Randomized trial of metformin, ivermectin and fluvoxamine for COVID-19. NEJM. 2022;387:599–610. doi: 10.1056/NEJMoa2201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy M., Naggie S., Boulware D.R., Lindsell C.J., Stweart T.G., Felker M. et al. Fluvoxamine for outpatient treatment of COVID-19: a decentralized, placebo-controlled, randomized, platform clinical trial. meRxiv https://doi.org/10.1101/2022.10.17.22281178
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this work are presented in the manuscript (Table 1, Fig. 1)