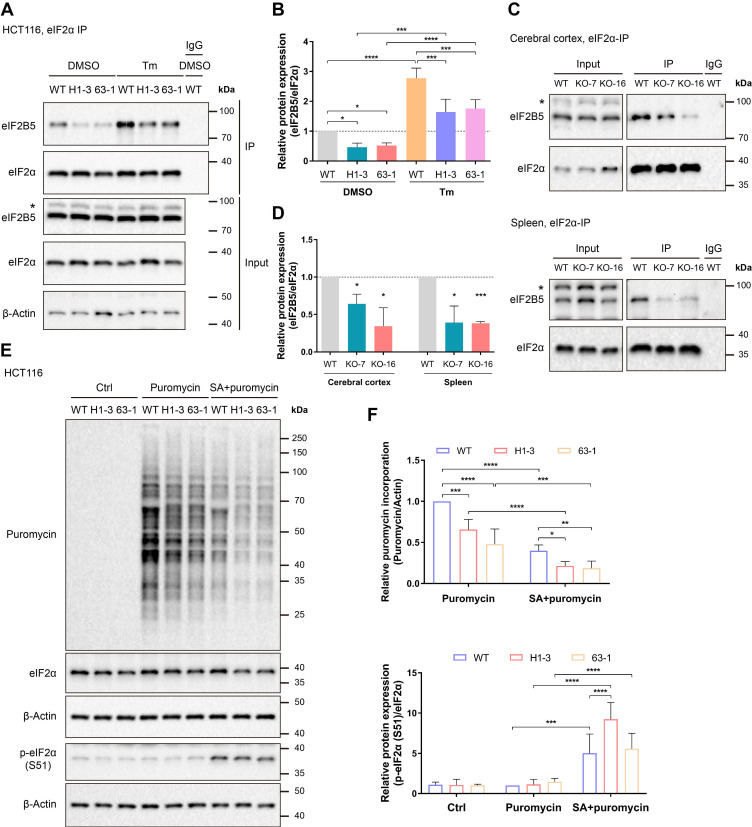
Figure 3.
Loss of C9orf72 weakens the interaction of eIF2α with eIF2B5 and inhibits global translation. (A) Lysates were prepared from either the DMSO- or Tm (1 μg/mL for 24 h)-treated WT or C9orf72-/- (H1-3 and 63-1) HCT116 cell lines. Endogenous eIF2α was immunoprecipitated with an eIF2α antibody pre-coupled to Protein A/G magnetic beads followed by western blot analyses using antibodies against eIF2α or eIF2B5. The asterisk (*) indicates a nonspecific band. (B) Relative ratio of eIF2B5 to eIF2α based on the western blot results (A) (n = 3 independent experiments, means ± SD, two-way ANOVA with Fisher's LSD test, *P ≤ 0.05, ***P ≤ 0.001, and ****P ≤ 0.0001). (C) Endogenous eIF2α in the cerebral cortex (top panel) or spleen (bottom panel) tissue from WT or C9orf72-null (KO-7 and KO-16) rats was immunoprecipitated with an eIF2α antibody pre-coupled to Protein A/G magnetic beads followed by western blot analyses using antibodies against eIF2α or eIF2B5. The asterisk (*) indicates a nonspecific band. (D) Relative ratio of eIF2B5 to eIF2α based on the western blot results (C) (n = 3 independent experiments, means ± SD, unpaired two-tailed t-test, *P ≤ 0.05 and ***P ≤ 0.001). (E) Western blot analysis of puromycin incorporation and eIF2α and p-eIF2α (S51) protein levels in the Ctrl group (untreated HCT116 cell lines, negative control), Puromycin group (HCT116 cell lines treated with 3 μg/mL puromycin for 30 min) and SA + puromycin group (HCT116 cell lines cotreated with 0.2 mM SA and 3 μg/mL puromycin for 30 min). β-Actin was used as a loading control. (F) Quantification of puromycin incorporation (upper panel) and the relative ratio of p-eIF2α (S51) to eIF2α (lower panel) based on the western blot results (E) (n = 4 independent experiments, means ± SD, two-way ANOVA with Fisher's LSD test, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001).